NCEA Level 1 Science/The structure of matter

Introduction
[edit | edit source]All matter is made up of very small particles called atoms. The name atom comes from the Greek meaning uncuttable, something that cannot be divided further. Atoms are the basic components of elements.
Subatomic Particles
[edit | edit source]
Atoms have three subatomic particles:
- protons (+): positively charged
- electrons (-): negatively charged
- neutrons (0): no charge
The can sometimes be written as (protons), (electrons) and (neutrons)
Normally, atoms have no overall charge (are neutral) because the number of positively charged protons equals the number of negatively charged electrons. The number of neutrons tends to vary and in the case of hydrogen, there are no neutrons. Atoms with the same number of protons but different masses are called isotopes.

Neutrons play a very important role in the functions of an atom. In the nucleus, several protons stay clumped together. As opposite charges attract and like charges repel, normally the protons would repel and scatter the nucleus. However, there is a strong nuclear force that holds the nucleus together. This incredible force causes nucleons (protons and neutrons) to attract each other with much greater strength than the electric force can repel them, but only over extremely short distances.

A delicate balance exists between the number of protons and neutrons. Protons, which are attracted to one another via the strong force but simultaneously repelled by their electromagnetic charges, cannot exist in great numbers within the nucleus without the stabilizing action of neutrons, which are attracted via the strong force but are not charged. Oppositely, neutrons lend their inherent instability to the nucleus and too many will destabilize it.
The shielding (or screening) effect is similar to effective nuclear charge. The core electrons repel the valence electrons (electrons on the outer shell) to a certain degree. The more electron shells there are (a new shell for each row in the periodic table), the greater the shielding effect is. Essentially, the core electrons shield the valence electrons from the positive charge of the nucleus.

Atomic Number
[edit | edit source]The atomic number is the number of protons in the nucleus of an atom. The atomic number determines what element the atom is. Elements on the periodic table are listed based on their atomic number. Each individual element has a unique identifying symbol.
If a heavy metal is bombarded with neutrons or a charged particle in an accelerometer and if there is an increase in the number of protons, then atomic number will increase also. This is the method of creating new artificial elements called the transuranic elements which are elements greater than 92 on the periodic table.
Mass Number
[edit | edit source]The mass number is the sum of protons plus neutrons in an atom.
| There is a difference between an atom's mass number and an element's atomic mass. The mass number measures the number of protons and neutrons in the nucleus of a particular atom. The atomic mass measures the average mass of all atoms for an element. For example, a carbon atom might have a mass number of 12 or 14 (or something else), but carbon in general has a mass of 12.011 amu. |
Magnesium’s atomic mass is 24.3051 but its mass number is 24.
Electron Arrangements
[edit | edit source]An element is a substance that contains only the same type of atom. That is, the atoms with the same number of protons.
Inside the nucleus, there are protons and neutrons. Electrons orbit the nucleus arranged in different energy levels or shells. For the first 20 elements, the maximum electron configuration for each shell is:
- 1st shell: 2
- 2nd shell: 8
- 3rd shell: 8
- 4th shell: 2 (the remaining electrons)
The electron arrangement tells us how many electrons can fit onto each shell.
- Examples
K (Potassium; atomic number: 19) has an electron configuration of 2,8,8,1.
This tells us that there are 2 electrons on the 1st shell, 8 on the 2nd, 8 on the 3rd and 1 on the 4th
Another way to calculate the electron configuration for the first 20 elements is by looking at the periodic table. The rows on the periodic table are called periods while the columns are called groups.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 1 H |
2 He | ||||||||||||||||
| 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||
| 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||
| 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
| 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| 55 Cs |
56 Ba |
57 La |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 87 Fr |
88 Ra |
89 Ac |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn | ||||||
| 58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
||||
| 90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr | ||||
To view a more complete periodic table, take a look at this periodic table. |
To calculate the electron arrangement, we can safely assume for the first 20 elements that the periods in which an element is found is equal to the number of shells it has. We can also assume that the number of elements in each period up to that shell is the number of electrons in each shell.
- Examples
Cl (Chlorine; atomic number: 17) is found on group 7 or 17 and is in period three. Thus it has 3 shells and 7 electrons on its last shell. Also, its electron configuration is 2,8,7.
Group 1, 2, 16, 17
[edit | edit source]Reactivity
[edit | edit source]
The group (column) in which an element is located can determine its reactivity. Elements in group 18 have full valence electrons thus they are unreactive or stable. These will not form compounds such as O2 or CO2. On the other hand, group 1 and 17 are very reactive because they have only one valence electron which is easily lost, thus group 1 and 17 elements are never found alone in nature - they will always be in compounds ie. H2 and Cl2. Group 2 and 16 are mildly reactive and are also naturally found as compounds.
Ions
[edit | edit source]| +1 | +2 | +3 | -2 | -1 |
|---|---|---|---|---|
| NH4+ | Ca 2+ | Al3+ | O2- | OH- |
| Na+ | Mg2+ | Fe3+ | S2- | Cl- |
| K+ | Cu2+ | CO32- | NO3- | |
| Ag + | Pb 2+ | SO42- | HCO3- | |
| H+ | Fe 2+ | |||
| Ba2+ | ||||
| Zn 2+ |
Ions are atoms or groups of atoms that have gained or lost electrons. The result is that they have become charge particles.
The periodic table is divided into two groups: metals and non-metals. Metals are found on the left while non-metals are found on the right. The elements in the center are metals but they are called transition metals. Metals become positive charged and are called cations. Non-metals become negatively charge and are called anions. Metals that lose one electrons will have more protons than electrons, so they form positive ions, e.g. H+ and Al3+. Whereas, non-metals that gain electrons will have more electrons than protons, so they form negative ions, eg. Cl- and HCO3-.
- In a chemical reaction, a hydrogen atoms loses its only electron to form an ion of 1 proton and 0 electrons. This Hydrogen ion (H+) has a charge of +1 because there is 1 proton while no electrons to balance the charge.
- A chlorine (2,8,7) atom gains an electron to form an ion of 17 protons and 18 electrons. This chloride ion (Cl-) has a charge of -1 because there are 17 protons while there are 18 electrons. The number of electrons is greater than the number of protons by one.
- Normally, a sodium atom (2,8,1) has 1 electron on its outer shell. In a chemical reaction, it will lose this electron to form a Na+ ion (2,8).
- Oxygen (2,6) in a chemical reaction will gain 2 electrons to complete its outer shell. It will form the ion O2+.
Atoms desire to have full valence energy level so it becomes unreactive and stable. This is done by gaining or losing electrons in a chemical reaction. A chemical reaction does not only occur in science labs. Group 1 and 17 are not naturally found in nature. Exposing a group 1 element like sodium to the atmosphere (which contains 78% nitrogen, 21% oxygen, 0.9% argon and 0.03% carbon dioxide) or specifically oxygen would create an immediate oxidisation reaction.
| Lose electrons | Positive ion |
|---|---|
| Gain electrons | Negative ion |
Ions and the Periodic Table
[edit | edit source]The reactivity and charge of an ions can be linked to its position on the periodic table.
- Group 1 metals have 1 electron on their outer shell therefore they are highly reactive and will lose this electron to form positive +1 ions, eg. Li+ and Na +.
- Group 2 metals have 2 electrons in their outer shell therefore they are mildly reactive and will lose these 2 electrons to form positive +2 ions, eg. Be2+ and Mg2+
- Group 16 non-metals have 6 electrons in their outer shell therefore they are mildly reactive and will gain 2 electrons to form a negative -2 ion, eg. O2-, S2-
- Group 17 non-metals have 7 electrons on their outer shell. They are highly reactive and will gain 1 electron to form a negative -1 ion, eg. F- , Cl-
- Group 18 non-metals have full outer shells. They are unreactive and do not form ions, eg. He, Ne, Ar
| Element | Electron Arrangement | Charge |
|---|---|---|
| Hydrogen | H +1 | |
| Helium | He 0 | |
| Lithium | Li +1 | |
| Beryllium | Be +2 | |
| Oxygen | O -2 | |
| Fluorine | F -1 | |
| Neon | Ne O |
Ionic Compounds
[edit | edit source]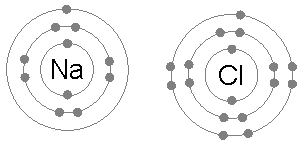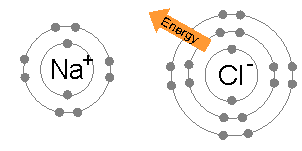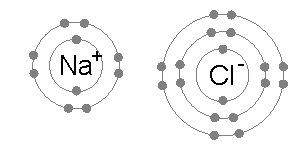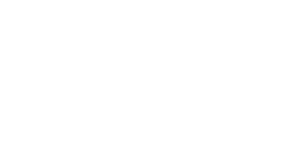
An ionic compound forms when a positive ion (metals) and a negative ion (non-metals) combine chemically. The process is reactant → product.
There are two types of equations:
- Word equation
- Chemical equation
A word equation involves identifying the reactants and the products. Chemical equations involve using words and symbols to write your equation. In the exam, be sure to read your question properly and write down the type of equation that is asked.
- Examples
- Word equation: Magnesium + Oxygen → Magnesium oxide
- Chemical Equation: Mg2+ + O2- → MgO
Naming and Writing Formulae
[edit | edit source]
To write ionic compounds, the drop and swap rule can be utilised.
Steps:
1. Find the ions on the table of ions, then remove the + and – signs
2. If the numbers are the same, they cancel each other out.
3. If the numbers are different, bring them across and drop them.
4. If a number is dropped to a compound, then brackets need to be inserted.
| Name of compound | Finding ionic charges | Numbers swapped and dropped |
|---|---|---|
| Magnesium Oxide | Mg2+ + O2- | MgO |
| Aluminium Hydroxide | Al3+ + OH- | (Al(OH))3 [Unbalanced] |
| Potassium Nitrate | K+ + NO3- | KNO3 |
| Calcium Chloride | Ca2+ + Cl- | CaCl2 [Unbalanced] |
| Zinc Nitrate | Zn2+ + NO3- | Zn[(NO3)]2 [Unbalanced] |
| Sodium Sulfate | Na+ + SO42- | Na2SO4 [Unbalanced] |
Compounds
[edit | edit source]
Compounds form when two or more elements combine together eg. CaCl2. Subscripted numbers within the formula tell you how many atoms of each element are present.
- Examples
-
- Ca(Cl)2 - 1 Ca, 2 Cl
- Mg(OH)2 - 1 Mg, 2 H, 2 O
Coefficients tell you how many molecules of that compound there are. To calculate the number of atoms of each element present, you multiply the coefficient by the atom number.
- Examples
-
- 3Cu(SO)4 - 3 Cu, 3 S, 12 O
- 2CO2 - 2 C, 4 O
- 5H2SO4 - 10 H, 5 S and 20 O
- 3Cu(OH)2 - 3 Cu, 6 O, 6 H




