9-1 Chemistry/Carbon compounds
A Brief Introduction to Organic chemistry
[edit | edit source]Crude oil, hydrocarbons and alkanes
[edit | edit source]Crude oil is a finite resource found in rocks. It comes from the remains of ancient biomass (mainly plankton) that was buried in mud.
Crude oil is a mixture of a very large number of compounds. Most of the compounds are hydrocarbons, molecules made up of only hydrogen and carbon atoms only. There are different forms of hydrocarbons but the most common type are known as alkanes. Alkanes are a homologous series; they have the similar properties and follow a certain pattern of chemical formula. The general formula for alkanes is:
This is demonstrated by the first 4 alkanes in the series:
- Methane
- Ethane
- Propane
- Butane
We can also draw a structural diagram for alkanes by writing the carbon bonds in the center and filling all the other sides with hydrogen atoms. For example, the diagram for butane would look like this:

Fractional distillation and petrochemicals
[edit | edit source]Although crude oil is a mixture of many different hydrocarbons, we are able to separate hydrocarbons into fractions with compounds that have similar numbers of carbon atoms, by fractional distillation. The fractionating column has a temperature gradient, with the column being hottest at the bottom and coolest at the top. The longest hydrocarbons drain out first, and the shortest hydrocarbons, which have the lower boiling point, drain out at the top.
The fractions can be processed to produce fuels and feedstock for the petrochemical industry. Many of the fuels that we depend on come from crude oil such as petrol, diesel oil, kerosene, heavy fuel oil and petroleum gases. These are used to produce many useful materials such as solvents, lubricants, polymers and detergents.
All of this is thanks to the amazing ability of carbon atoms to form families of similar compounds (homologous series).
Properties of hydrocarbons
[edit | edit source]
Smaller hydrocarbons, by molecular size, have:
- higher viscosity (flow more easily)
- are more volatile
- have a lower boiling point
- and have a higher flammability (ignite more easily).
Longer hydrocarbons have:
- a higher boiling point
- lower viscosity
- not very volatile
- and low flammability.
These properties result in hydrocarbon fractions being used very differently. The longest hydrocarbon, bitumen, is used for road tarmac for example. Also, longer hydrocarbons are used as fuels for larger vehicles or systems such as heavy fuel oil being used for power stations and ships whilst shorter hydrocarbons such as petrol, is used as fuel for cars. The smallest hydrocarbon, liquified petroleum gas, is used as fuel for cooking and heating a house.
Combustion of hydrocarbons
[edit | edit source]Hydrocarbon fuels are burnt to release energy. This is known as combustion, and all combustion reactions are exothermic; they give out energy to the environment. When there is a good supply of oxygen (from the air) then combustion reactions are complete and follow this pattern:
This reaction provides the maximum amount of energy and is known as complete combustion. The following is a chemical equation for the complete combustion of ethane:
Combustion reactions are the only time in the chemistry course you can get away with half of a molecule, or alternatively you could double everything. In incomplete combustion carbon monoxide is produced instead of carbon dioxide:
Cracking and alkenes
[edit | edit source]
Cracking is when hydrocarbons are broken down into smaller molecules which are often more useful. Cracking is also an example of a thermal decomposition reaction where molecules are broken down by heating.
Catalytic cracking is when a catalyst is used to provide an alternative reaction pathway, lowering the temperature (around 500 Degrees Celsius) and pressure required for hydrocarbons to break down. The catalyst used is often zeolite which contains aluminium oxide and silicon oxide. On the other hand, steam (thermal) cracking does not use a catalyst and therefore requires a higher temperature (around 800 Degrees Celsius) and pressure. Catalytic cracking is much cheaper so is the preferred method for cracking hydrocarbons.
The products of cracking include smaller alkanes and another type of hydrocarbon known as alkenes:
Alkenes follow the same prefix (beginning bit of word) as alkanes however instead of -ane they have -ene. Ethene is the smallest alkene (there is no such thing as methene!).
The general formula for alkenes is:
If you recall, shorter alkene hydrocarbons are used for fuels of smaller vehicles such as cars, so smaller there is a far higher fuel demand for shorter hydrocarbons. Alkenes are used to produce polymers and as starting materials for many other chemicals.
Drawing alkenes
[edit | edit source]All alkenes have a carbon double covalent bond, , whilst alkanes only have single bonds. Here is the diagram for ethene:
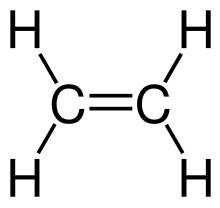
To draw larger alkenes:
- Cut off one side of the hydrogens from one side of the of ethene.
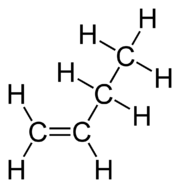
- Draw out the number of carbon atoms and bond each together such as for butene: (Remember that the left end should still have the 2 hydrogens)
- Fill all the sides with hydrogens except:
- There should only be 1 hydrogen to the other carbon in the double bond.
- There should be a hydrogen at the end.
This is what butene's displayed formula looks like:
Test for alkenes
[edit | edit source]Alkenes are more reactive than alkanes, and therefore react with bromine water, an orange solution of bromine, by decolourising it into a colourless solution. Alkanes do not.
Quick Questions
[edit | edit source]| What is the definition of Energy? |
| The maximum amount of change possible in a system (object or group of objects) |
| What are the 2 main types of energy store and what is the difference between them? |
| Energy stores that relate to movement (Kinetic) and Potential energy stores which relate to the position of an object in space. |
| What is energy measured in? |
| Joules |
| What is specific heat capacity? |
| The amount of energy it takes to increase 1 Kg of a substance by 1 |
| What is Power? |
| The rate of energy transfer |













