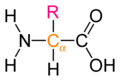A-level Chemistry/OCR (Salters)/Amino acids
Appearance
α-Amino acids
[edit | edit source]-
The general structure of an α-amino acid. The α-carbon is displayed in orange, and the side chain is denoted by R. This style of diagram does not indicate whether the amino acid is an L or a D enantiomer.
-
The stereochemistry of an L-amino acid. All amino acids in nature are L-amino acids, except for bacterial cell walls and the odd w:en:cone snail or two.
-
A skeletal formula of a general α-amino acid. The α-carbon is displayed as an orange circle
-
The skeletal formula of a general L-α-amino acid, the enantiomeric form found in nature.
-
The skeletal formula of a general D-α-amino acid, the enantiomeric form not found in nature. D-amino acids can be synthesised artificially in a laboratory.
The twenty protein-forming amino acids
[edit | edit source]-
glycine
Gly, G -
L-alanine
Ala, A -
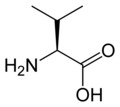
L-valine
Val, V -
L-leucine
Leu, L -
L-isoleucine
Ile, I -
L-phenylalanine
Phe, F -
L-proline
Pro, P -
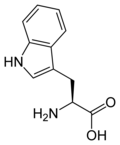
L-tryptophan
Trp, W -
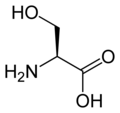
L-serine
Ser, S -
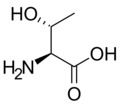
L-threonine
Thr, T -
L-cysteine
Cys, C -
L-methionine
Met, M -
L-aspartic acid
Asp, D -
L-glutamic acid
Glu, E -
L-asparagine
Asn, N -
L-glutamine
Gln, Q -
L-tyrosine
Tyr, Y -
L-histidine
His, H -
L-lysine
Lys, K -
L-arginine
Arg, R
Reactions of amino acids
[edit | edit source]Amino acids can undergo condensation reactions, forming polypeptides.