A-level Chemistry/OCR (Salters)/Penicillins
The penicillin nucleus
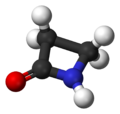
[edit | edit source]The pharmacophore (part of a molecule that confers pharmacological activity) of penicillins is called the penicillin nucleus. It contains a β-lactam ring.
The β-lactam ring
[edit | edit source]The penicillin nucleus found in all penicillins contains a so-called β-lactam ring. This is a four-membered cyclic amide that is unstable in acidic or alkaline conditions. The small ring is under a great deal of strain, making the normally unreactive amide group more susceptible to hydrolysis, resulting in ring-opening to form open-chain compounds.
-
skeletal formula of the penicillin nucleus -
ball-and-stick model of the penicillin nucleus -
ball-and-stick model of the β-lactam ring -
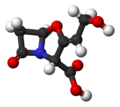
ball-and-stick model of clavulanic acid
β-lactamase inhibitors
[edit | edit source]β-Lactamase is an enzyme produced by certain penicillin-resistant bacteria. It inactivates penicillin antibiotics by disrupting their β-lactam ring. Once way to protect penicillins against β-lactamase is to modify their side chains − methicillin and flucloxacillin are examples of penicillins whose side chains make them β-lactamase resistant. Another tactic is to protect vulnerable penicillins with a β-lactamase inhibitor. These compounds, such as clavulanic acid, are not antibiotics, but they allow penicillin antibiotics to do their job by inhibiting β-lactamase.
Table of penicillins
[edit | edit source]| Name | R group in side-chain | Natural/semi-synthetic | Uses/properties | Skeletal formula | 3D model |
|---|---|---|---|---|---|
| benzylpenicillin penicillin G |
natural | general infections, gonorrhoea and syphilis | 
|

| |
| flucloxacillin | semi-synthetic | controlling resistant Staphylococcus | 
|

| |
| penicillin F | natural | not used commercially | 
|
||
| penicillin X | natural | not used commercially | 
|
||
| penicillin K | natural | not used commercially | 
|
||
| penicillin V | natural and semi-synthetic | general infections, ear, nose and throat | 
|
||
| methicillin | semi-synthetic | controlling resistant Staphylococcus | 
|
||
| ampicillin | semi-synthetic | lung and wound infections | 
|
||
| amoxycillin | semi-synthetic | lung and urinary tract infections | 
|

| |
| carbenicillin | semi-synthetic | pneumonia, burns | 
|