A-level Chemistry/OCR (Salters)/Spectroscopy
Spectroscopy is the study of the interaction between radiation (electromagnetic radiation or particle radiation) and matter. In Chemical Ideas, the whole of Chapter 6: Radiation and matter is devoted to spectroscopy. It covers both how and why radiation interacts with matter, and how such interactions are applied.
There are several different spectroscopic techniques discussed in Salters Advanced Chemistry. They are:
- infrared spectroscopy (IR)
- nuclear magnetic resonance spectroscopy (NMR)
- ultraviolet-visible spectroscopy (UV-Vis)
- mass spectrometry (MS)
In addition, laser microspectral analysis (LMA) is briefly mentioned in the Chemical Storylines chapter Colour by Design.
There are many other kinds of spectroscopy used by scientists every day, but the ones mentioned here are the most important to chemistry as a whole.
Colour
[edit | edit source]Why are substances coloured?
[edit | edit source]We'll consider the major reason for the colour of substances: absorption of light. There are other ways in which a substance can exhibit colour — it can scatter or emit light, for instance — but these effects are best discussed elsewhere.
The reason substances are coloured is usually that they absorb some energies of visible light, but transmit or reflect the rest. All energies except those absorbed are detected in our eyes. Our brains then interpret this distribution of photons of different energies as a particular colour.
The colour wheel helps predict how our brains interpret different distributions of photon energy. White light (e.g. sunlight) contains photons of every possible energy, i.e. the entire visible spectrum. This distribution of photon energies stimulates the eyes and brain in a way that we perceive as white.
-
the colour wheel -
a blue solution absorbs orange light
-
a colourless solution transmits all visible light
What is light?
[edit | edit source]When we talk about light in science, we usually mean electromagnetic radiation in all its forms. This includes not just visible light, but the entire electromagnetic spectrum, covering everything from γ-rays to radio waves.
Electromagnetic radiation consists of particles called photons. Any particular photon has a specific amount of energy, which determines its frequency and wavelength.
Why do all substances absorb electromagnetic radiation?
[edit | edit source]All substances have electrons in them, and electrons occupy energy levels. All substances also contain vacant energy levels that are capable of containing an electron, but are not occupied.
An electron in a substance can move from the energy level it occupies to a vacant energy level that is higher in energy. This move requires the electron to acquire a precise quantity of energy. The electron can absorb a photon if the photon has that exact amount of energy. By absorbing the photon, the electron increases in energy by exactly the right amount and moves to the vacant energy level.
Why do some substances absorb visible light?
[edit | edit source]Substances that absorb visible light are ones that have two energy levels whose energy difference is in the visible range, 2.8 × 10−19 J − 5.0 × 10−19 J. Such substances can absorb photons of this energy range.
A photon whose energy is between 2.8 × 10−19 J − 5.0 × 10−19 J has a wavelength between 700 nm and 400 nm and a frequency between 4.3 × 1014 Hz and 7.5 × 1014 Hz.
In a nutshell,
Why do most transition metal complexes absorb visible light?
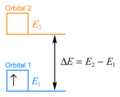
[edit | edit source]In transition metal complexes, we can define what we mean by energy levels more carefully. By energy levels, we mean orbitals. Electrons in a transition metal complex can be moved from their normal orbital to a higher-energy orbital.
In its normal orbital, call it Orbital 1, an electron has a particular amount of energy, .
In the higher-energy orbital, call it Orbital 2, the electron has a greater amount of energy, .
The energy difference between the two situations is .
-
Orbital 1 is occupied by an electron, while Orbital 2 is vacant -
The electron absorbs a photon with the correct energy and is promoted from Orbital 1 to Orbital 2 -
Orbital 1 is now vacant and Orbital 2 is occupied
When a photon arrives at Orbital 1, the electron may be able to absorb the photon.
If , the photon does not have enough energy to promote the electron from Orbital 1 to Orbital 2, so the electron cannot absorb the photon.
If , the photon has too much energy to promote the electron from Orbital 1 to Orbital 2, so the electron cannot absorb the photon.
If , the photon has exactly the right amount of energy to promote the electron from Orbital 1 to Orbital 2. The electron can and does absorb the photon. The electron acquires the photon's energy and is promoted from Orbital 1 to Orbital 2.