A-level Chemistry/OCR (Salters)/Sulfur
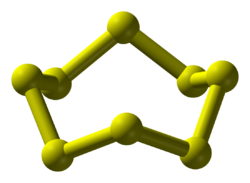
Sulfur is the sixteenth element in the periodic table, lying below oxygen and above selenium in Group 16. Sulfur is in Period 3, with phosphorus to its left and chlorine to its right. Sulfur exists as a yellow solid, which contains eight-membered rings stacked together. This is in stark contrast with oxygen, which exists as the molecular gas dioxygen, O2, but similar to phosphorus and selenium.
Isotopes
[edit | edit source]95% of naturally occurring sulfur is sulfur-32, 32S. Most of the rest is sulfur-34, 34S. Two other stable isotopes exist, 33S and 36S. Sulfur's relative atomic mass is 32.065 ± 0.005.
Electron configuration
[edit | edit source]The electron configuration of the neutral sulfur atom, S, is
or in more compact notation,
Electronegativity
[edit | edit source]Sulfur has an electronegativity of about 2.6, which is similar to carbon and selenium. Its electronegativity is greater than that of any metal or metalloid, and greater than that of the nonmetal phosphorus (2.2). The other non-metals (I, Br, Cl, N, O and F) are more electronegative.
Compounds
[edit | edit source]Sulfides
[edit | edit source]Sulfur forms sulfides by direct reaction with metals:
- Fe(s) + S(s) → FeS(s)
- Zn(s) + S(s) → ZnS(s)
- 2Li(s) + S(s) → Li2S(s)
- 2Al(s) + 3S(s) → Al2S3(s)
Sulfides formally contain the sulfide ion, S2−, but the bonding in sulfides is somewhere in-between ionic and covalent, with some sharing of electrons between metal and sulfur. S2− is almost nonexistent in solution, because it is extremely basic and will remove a proton from almost anything. Consequently, sulfides are insoluble in all solvents, unless they react with the solvent to form something more soluble.
Organosulfur compounds
[edit | edit source]- Sulfonic acids, RSO3H and sulfonates, RSO3−, covered in Colour by Design
- Thioethers (sulfides), e.g. dimethyl sulfide, (CH3)2S, mentioned in The Oceans
- Many other organic compounds containing sulfur exist, but they are not discussed in Salters Advanced Chemistry


![{\displaystyle [{\mbox{Ne}}]3{\mbox{s}}^{2}3{\mbox{p}}^{4}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/aabddf0f6830ea6077c91b0547b85df4e01cbe00)