AQA A-Level Physics/Atomic structure
The atom as we know it, was not originally known as it is today. As you may know from GCSE physics, the way in which an atom is structured consists of a nucleus and electrons. This isn't far from the truth, but there are some differences in the way in which the atom is laid out. To better understand this, we need to look at how the modern structure of the atom was discovered.
10.1.1 Constituents of the atom -- "What is the atom made of"
[edit | edit source]Rutherford Scattering
[edit | edit source]
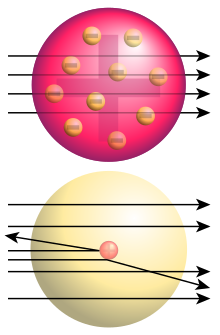
Originally the atom was thought of as a tiny piece of solid matter. People knew that the atom was the smallest thing you could have; the word atom comes from a Greek word meaning indivisible. So if the atom was the smallest thing it seemed perfectly sensible to imagine it as a sort of solid jelly.
Rutherford had a different idea, and to test it he had to make a very thin solid layer. He used gold, because it is easier to roll or hammer out a thin solid layer of gold than any other material. He wanted a thin layer because he wondered if the atoms really were solid.
To test his idea he fired alpha particles (usually written as -particles) at the foil. These particles consist of 2 protons and 2 neutrons, that is a helium nuclei. They were quite easy to use because a common type of radio-active decay emits -particles. When this experiment was first performed, by Rutherford's assistants, Geiger and Marsden, in 1909 they found a rather interesting result. Most of the particle went straight through the foil. However, a few of them bounced off. This behaviour could not be explained if the atoms are really a jelly-like solid.
If we think about this result and consider it at the large scale we can get an idea of what is happening. Imagine we have a wire fence, and let's imagine that it is perfectly strong, and we fire balls at it. Let this fence be the nice open net that we often get with holes about 75mm square. If we fire a football we are not surprised if it just bounces straight off. The football is much bigger than the holes in the fence and so the fence appears to be solid. If we hit golf balls at the fence we'd not be surprised if most went straight through it, and the ones that hit right onto a wire bounced back. If we fired slugs from an air pistol we'd expect even more to go straight through.
This sort of thinking gave Rutherford the idea of the atom.
- He suggested that because most of the -particles went straight through the atom must contain a majority of empty space.
- Some alpha particles scattered off so these must have been repelled by something.
- That something must have a positive charge to repel the positive charge of the -particle and must be heavier than the -particle to make it bounce the way that it did.
This gave Rutherford the idea that the atom was mostly empty space, with a heavy, positively charged nucleus with the relatively light, negatively-charged electrons orbiting around it like planets around a star. (Of course, in those days the only star that we knew had planets was our sun.)
The now-known structure
[edit | edit source]As we know now, the atom contains:
- Nucleons (Protons and Neutrons bundled together)
- Electrons
Now, the things to remember about atoms is that The atom is defined by the number of protons in the nucleus, so if there's 1 proton in the nucleus, then it's going to be hydrogen, because its atomic number (number of protons) is 1. If you want to know what the element is, look it up on the periodic table. Now, for some quick facts:
- The number of electrons in an atom is equal to the number of protons, due to the charge of 1 proton pulling in 1 electron (in AS anyway)
- When electrons are removed or added to an atom, it becomes an ion. This is called Ionisation.
- When there are is a different no. of neutrons than usual in the nucleus, then an atom is an Isotope.
- Isotopes have the same physical and chemical properties, but the nuclei can be either stable or unstable, for example, C-12 and C-14 both occur in matter but the C-14 isotope is unstable
These are important concepts, as they're the basis of other theories and models that you'll learn later on in the module. Now, remember these definitions.
- Isotopes are atoms of the same element with different masses due to differing numbers of neutrons in their nucleus.
- Ions are atoms which have a number of electrons different to the number of protons, resulting in a charge.
| A | X |
| Z |
| 4 | He |
| 2 |
To understand what these mean, you need to know what the top value and the bottom value means.. the top value,
- A is the number of protons AND neutrons in the nucleus of the element, known as nucleon number
- Z is the number of protons in the nucleus.. so, therefore...
- number of neutrons.
Now, with that said, you will need to be able to calculate the masses and charges of these particles, and you will need to use their specific values unlike in GCSE. Don't worry, you don't need to remember them as you will get them in a data sheet at the front of the exam paper. With that said, it won't hurt to remember them!
| Particle | Charge | Mass |
| Proton | ||
| Neutron | NONE | |
| Electron |
Practice Questions
[edit | edit source]Don't let the wording phase you, and make sure to read and understand what answer the question wants, and what part is just explaining something. To see the answers, look below.
- An isotope of Plutonium-210 is a radioactive isotope, which emits alpha radiation. Calculate:
- The number of protons
- The number of electrons
- The number of neutrons
- The isotope undergoes an ionisation process which removes 4 electrons from the atom. Calculate the overall charge of the atom.
Answers
[edit | edit source]- Number of protons= 92, Number of neutrons=116, number of electrons=94
- (2 x 1.60 x10−19) = + 3.2 x10−19






