Basic Physics of Nuclear Medicine/Atomic & Nuclear Structure

You will have encountered much of what we will cover here in your high school physics. We are going to review this material again below so as to set the context for subsequent chapters. This chapter will also provide you with an opportunity to check your understanding of this topic.
The chapter covers atomic structure, nuclear structure, the classification of nuclei, binding energy and nuclear stability.
Atomic Structure
[edit | edit source]The atom is considered to be the basic building block of all matter. Simple atomic theory tells us that it consists of two components: a nucleus surrounded by an electron cloud. The situation can be considered as being similar in some respects to planets orbiting the sun.
From an electrical point of view, the nucleus is said to be positively charged and the electrons negatively charged.
From a size point of view, the radius of an atom is about 10-10 m while the radius of a nucleus is about 10-14 m, i.e. about ten thousand times smaller. The situation could be viewed as something like a cricket ball, representing the nucleus, in the middle of a sporting arena with the electrons orbiting somewhere around where the spectators would sit. This perspective tells us that the atom should be composed mainly of empty space. However, the situation is far more complex than this simple picture portrays in that we must also take into account the physical forces which bind the atom together.
Chemical phenomena can be thought of as interactions between the electrons of individual atoms. Radioactivity on the other hand can be thought of as changes which occur within the nuclei of atoms.
The Nucleus
[edit | edit source]A simple description of the nucleus tells us that it is composed of protons and neutrons. These two particle types are collectively called nucleons, i.e. particles which inhabit the nucleus.
From a mass point of view the mass of a proton is roughly equal to the mass of a neutron and each of these is about 2,000 times the mass of an electron. So most of the mass of an atom is concentrated in the small region at its core.
From an electrical point of view the proton is positively charged and the neutron has no charge. An atom all on its own (if that were possible to achieve!) is electrically neutral. The number of protons in the nucleus of such an atom must therefore equal the number of electrons orbiting that atom.
Classification of Nuclei
[edit | edit source]The term Atomic Number is defined in nuclear physics as the number of protons in a nucleus and is given the symbol Z. From your chemistry you will remember that this number also defines the position of an element in the Periodic Table of Elements.
The term Mass Number is defined as the number of nucleons in a nucleus, that is the number of protons plus the number of neutrons, and is given the symbol A.
Note that the symbols here are a bit odd, in that it would prevent some confusion if the Atomic Number were given the symbol A, and the Mass Number were given another symbol, such as M, but its not a simple world!
It is possible for nuclei of a given element to have the same number of protons but differing numbers of neutrons, that is to have the same Atomic Number but different Mass Numbers. Such nuclei are referred to as Isotopes. All elements have isotopes and the number ranges from three for hydrogen to over 30 for elements such as caesium and barium.
Chemistry has a relatively simple way of classifying the different elements by the use of symbols such as H for hydrogen, He for helium and so on. The classification scheme used to identify different isotopes is based on this approach with the use of a superscript before the chemical symbol to denote the Mass Number along with a subscript before the chemical symbol to denote the Atomic Number. In other words an isotope is identified as:
where X is the chemical symbol of the element; A is the "Mass Number," (protons+ neutrons); Z is the "Atomic Number," (number identifying the element on the periodic chart).
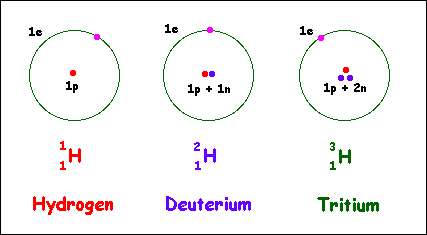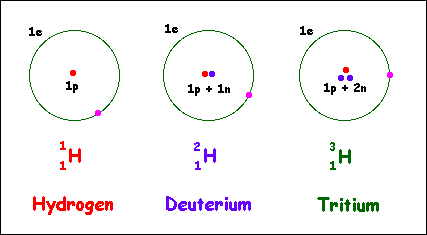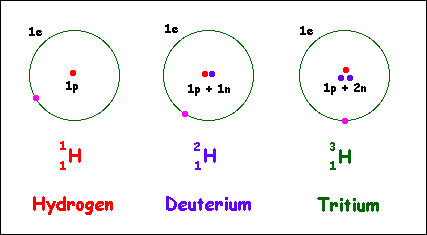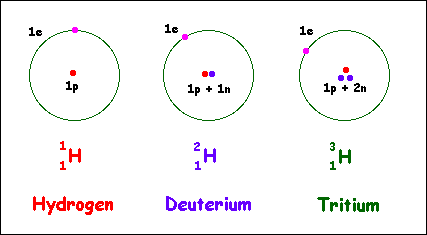
Let us take the case of hydrogen as an example. It has three isotopes:
- the most common one consisting of a single proton orbited by one electron,
- a second isotope consisting of a nucleus containing a proton and a neutron orbited by one electron,
- a third whose nucleus consists of one proton and two neutrons, again orbited by a single electron.
A simple illustration of these isotopes is shown below. Remember though that this is a simplified illustration given what we noted earlier about the size of a nucleus compared with that of an atom. But the illustration is nevertheless useful for showing how isotopes are classified.
The first isotope commonly called hydrogen has a Mass Number of 1, an Atomic Number of 1 and hence is identified as:
The second isotope commonly called deuterium has a Mass Number of 2, an Atomic Number of 1 and is identified as:
The third isotope commonly called tritium is identified as:
The same classification scheme is used for all isotopes. For example, you should now be able to figure out that the uranium isotope, , contains 92 protons and 144 neutrons.
A final point on classification is that we can also refer to individual isotopes by giving the name of the element followed by the Mass Number. For example, we can refer to deuterium as hydrogen-2 and we can refer to as uranium-236.
Before we leave this classification scheme let us further consider the difference between chemistry and nuclear physics. You will remember that the water molecule is made up of two hydrogen atoms bonded with an oxygen atom. Theoretically if we were to combine atoms of hydrogen and oxygen in this manner many, many of billions of times we could make a glass of water. We could also make our glass of water using deuterium instead of hydrogen. This second glass of water would theoretically be very similar from a chemical perspective. However, from a physics perspective our second glass would be heavier than the first since each deuterium nucleus is about twice the mass of each hydrogen nucleus. Indeed water made in this fashion is called heavy water.
Atomic Mass Unit
[edit | edit source]The conventional unit of mass, the kilogram, is rather large for use in describing characteristics of nuclei. For this reason, a special unit called the Atomic Mass Unit (amu) is often used. This unit is sometimes defined as 1/12th of the mass of the stable most commonly occurring isotope of carbon, i.e. 12C. In terms of grams, 1 amu is equal to 1.66 x 10-24 g, that is, just over one million, million, million millionth of a gram.
The masses of the proton, mp and neutron, mn on this basis are:
and
while that of the electron is just 0.00055 amu.
Binding Energy
[edit | edit source]We are now in a position to consider the subject of nuclear stability. From what we have covered so far, we have seen that the nucleus is a tiny region in the centre of an atom and that it is composed of neutrally and positively charged particles. So, in a large nucleus such as that of uranium (Z=92) we have a large number of positively charged protons concentrated into a tiny region in the centre of the atom. An obvious question which arises is that with all these positive charges in close proximity, why doesn't the nucleus fly apart? How can a nucleus remain as an entity with such electrostatic repulsion between the components? Should the orbiting negatively-charged electrons not attract the protons away from the atoms centre?
Let us take the case of the helium-4 nucleus as an example. This nucleus contains two protons and two neutrons so that in terms of amu we can figure out from what we covered earlier that the
and the
Therefore we would expect the total mass of the nucleus to be 4.03298 amu.
The experimentally determined mass of a helium-4 nucleus is a bit less - just 4.00260 amu. In other words there is a difference of 0.03038 amu between what we might expect as the mass of this nucleus and what we actually measure. You might think of this difference as very small at just 0.75%. But remember that since the mass of one electron is 0.00055 amu the difference is actually equivalent to the mass of about 55 electrons. Therefore it is significant enough to wonder about.
It is possible to consider that this missing mass is converted to energy which is used to hold the nucleus together; it is converted to a form of energy called Binding Energy. You could say, as with all relationships, energy must be expended in order to maintain them!
Like the gram in terms of the mass of nuclei, the common unit of energy, the joule is rather cumbersome when we consider the energy needed to bind a nucleus together. The unit used to express energies on the atomic scale is the electron volt, symbol: eV.
One electron volt is defined as the amount of energy gained by an electron as it falls through a potential difference of one volt. This definition on its own is not of great help to us here and it is stated purely for the sake of completeness. So do not worry about it for the time being. Just appreciate that it is a unit representing a tiny amount of energy which is useful on the atomic scale. It is a bit too small in the case of binding energies however and the mega-electron volt (MeV) is often used.
Albert Einstein introduced us to the equivalence of mass, m, and energy, E, at the atomic level using the following equation:
where c is the velocity of light.
It is possible to show that 1 amu is equivalent to 931.48 MeV. Therefore, the mass difference we discussed earlier between the expected and measured mass of the helium-4 nucleus of 0.03038 amu is equivalent to about 28 MeV. This represents about 7 MeV for each of the four nucleons contained in the nucleus.
Nuclear Stability
[edit | edit source]In most stable isotopes the binding energy per nucleon lies between 7 and 9 MeV. There are two competing forces in the nuclei, electrostatic repulsion between protons and the attractive nuclear force between nucleons (protons and neutrons). The electrostatic force is a long range force that becomes more difficult to compensate for as more protons are added to the nucleus. The nuclear force, which arises as the residual strong force (the strong force binds the quarks together within a nucleon), is a short range force that only operates on a very short distance scale (~ 1.5 fm) as it arises from a Yukawa potential. (Electromagnetism is a long range force as the force carrier, the photon, is massless; the nuclear force is a short range force as the force carrier, the pion, is massive). Therefore, larger nuclei tend to be less stable, and require a larger ratio of neutrons to protons (which contribute to the attractive strong force, but not the long-range electrostatic repulsion). For the low Z nuclides the ratio of neutrons to protons is approximately 1, though it gradually increases to about 1.5 for the higher Z nuclides as shown below on the Nuclear Stability Curve.

In other words to combat the effect of the increase in electrostatic repulsion when the number of protons increases the number of neutrons must increase more rapidly to contribute sufficient energy to bind the nucleus together.
As we noted earlier there are a number of isotopes for each element of the Periodic Table. It has been found that the most stable isotope for each element has a specific number of neutrons in its nucleus. Plotting a graph of the number of protons against the number of neutrons for these stable isotopes generates what is called the Nuclear Stability Curve:
Note that the number of protons equals the number of neutrons for small nuclei. But notice also that the number of neutrons increases more rapidly than the number of protons as the size of the nucleus gets bigger so as to maintain the stability of the nucleus. In other words more neutrons need to be there to contribute to the binding energy used to counteract the electrostatic repulsion between the protons.
Radioactivity
[edit | edit source]There are about 2,450 known isotopes of the approximately one hundred elements in the Periodic Table. You can imagine the size of a table of isotopes relative to that of the Periodic Table! The unstable isotopes lie above or below the Nuclear Stability Curve. These unstable isotopes attempt to reach the stability curve by splitting into fragments, in a process called Fission, or by emitting particles and/or energy in the form of radiation. This latter process is called Radioactivity.
It is useful to dwell for a few moments on the term radioactivity. For example what has nuclear stability to do with radio? From a historical perspective remember that when these radiations were discovered about 100 years ago we did not know exactly what we were dealing with. When people like Henri Becquerel and Marie Curie were working initially on these strange emanations from certain natural materials it was thought that the radiations were somehow related to another phenomenon which also was not well understood at the time - that of radio communication. It seems reasonable on this basis to appreciate that some people considered that the two phenomena were somehow related and hence that the materials which emitted radiation were termed radio-active.
We know today that the two phenomena are not directly related but we nevertheless hold onto the term radioactivity for historical purposes. But it should be quite clear to you having reached this stage of this chapter that the term radioactive refers to the emission of particles and/or energy from unstable isotopes. Unstable isotopes for instance those that have too many protons to remain a stable entity are called radioactive isotopes - and called radioisotopes for short. The term radionuclide is also sometimes used.
Finally about 300 of the 2,450-odd isotopes mentioned above are found in nature. The rest are man-made, that is they are produced artificially. These 2,150 or so artificial isotopes have been made during the last 100 years or so with most having been made since the second world war.
We will return to the production of radioisotopes in a later chapter of this wikibook and will proceed for the time being with a description of the types of radiation emitted by radioisotopes.
Multiple Choice Questions
[edit | edit source]Click here to access multiple choice questions on atomic and nuclear structure.
External Links
[edit | edit source]- Novel Periodic Table - an interactive table providing information about each element.
- Marie and Pierre Curie and the Discovery of Polonium and Radium - an historical essay from The Nobel Foundation.
- Natural Radioactivity - an overview of radioactivity in nature - includes sections on primordial radionuclides, cosmic radiation, human produced radionuclides, as well as natural radioactivity in soil, in the ocean, in the human body and in building materials - from the University of Michigan Student Chapter of the Health Physics Society.
- The Particle Adventure - an interactive tour of the inner workings of the atom which explains the modern tools physicists use to probe nuclear and sub-nuclear matter and how physicists measure the results of their experiments using detectors - from the Particle Data Group at the Lawrence Berkeley National Lab, USA and mirrored at CERN, Geneva.
- WebElements - an excellent web-based Periodic Table of the Elements which includes a vast array of data about each element - originally from Mark Winter at the University of Sheffield, England.