Biochemistry/Metabolism and energy
Metabolism
[edit | edit source]Anabolism and catabolism
[edit | edit source]Metabolism (Fig. 1) is, broadly speaking, the conversion of food into energy, cell components, and waste products.

Figure 1: Overview of metabolism
The above diagram shows the different parts of metabolism:
- energy source, which is, after all, the sun, whose energy is harvested through photosynthesis
- catabolism, the breakdown of food into chemical energy, which is needed in
- anabolism, the construction of complex cell molecules from small environmental molecules, utilizing chemical energy
Catabolic reactions release energy and are therefore exergonic, while anabolic reactions use up energy and are therefore endergonic.
High-energy phosphates
[edit | edit source]Due to the large variety of food compounds, and the large number of biochemical reactions which need energy in anabolism, it would be quite inefficient to couple a specific anabolic reaction to a specific energy source in catabolism. Instead, the cell uses an intermediate compound, a kind of universal energy currency. This intermediate is called high-energy phosphate.
But when is a phosphate group called "high-energy", and how does it differ from a "low-energy" phosphate? A giveaway is the ΔG0' of hydrolysis. Hydrolysis separates a phosphate from a compound by adding water:
O O R-OP-OH + H2O ⇌ R-OH + HO-P-OH O O
The ΔG0' of a low-energy (or "inorganic") phosphate group (called Pi) is 9-20 kJ mol-1, while the ΔG0' of a high-energy phosphate (denoted Ⓟ) is ~30 kJ mol-1.
pKa value
[edit | edit source]Now what makes this Ⓟ so special? To explain this, we must take a little excourse into pH and pKa values. A phosphate group has between zero and three OH groups. This allows Ⓟ to exist in up to four different forms (0, 1, 2, and 3 OH groups, Fig. 2), depending on the pH value of the surrounding solution. A pKa value gives us the pH value at which 50% of the molecules are in one form (e.g., 1 OH group) and another (e.g., 2 OH groups). This is expressed by the Henderson-Hasselbalch equation :

Figure 2: The four possible forms of a phosphate group. pKa2 represents the conditions in the cell.
Now to the promised difference between Ⓟ and PPi. The breaking of the ester bond of an ROⓅ releases more energy than the breaking of a PPi bond (Fig. 3), because of
- electrostatic repulsion between the two phosphate groups in PPi
- resonance stabilization of two Pi groups, compared to PPi (Fig. 4)

Figure 3: Hydrolysis of Ⓟ and PPi.

Figure 4: Resonance stabilization of Pi.
Resonance stabilization means that both OH and =O can "travel" around the phosphate. Of course, this is a crude analogy; they do not really move, the electrons are just "smeared" around the phosphate atom. This is also indicated by the use of the ↔ arrow, instead of ⇌; the three forms do not exist, they are just a way of writing down the chemical reality.
As you can see in Fig. 3, the ΔG0' value for PPi⇌2Pi is ≪0, shifting the reaction strongly in favor of the 2Pi.
Molecules using high-energy phosphates
[edit | edit source]Anhydride between phosphoric acid and carboxyl group
[edit | edit source]Hydrolysis : ΔG0' = -49.3 kJ mol-1

Guanidine phosphate
[edit | edit source]Hydrolysis : ΔG0' = -43.0 kJ mol-1

Enol phosphate
[edit | edit source]In the below picture, the final product should not have a carbon-carbon double bond, but a single bond with CH3 on the top. It is an error.
Hydrolysis : ΔG0' = -61.9 kJ mol-1
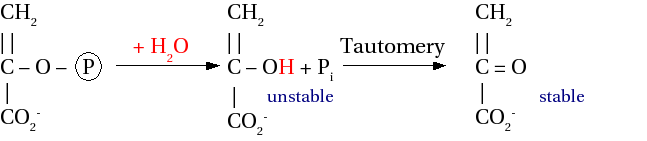
ATP
[edit | edit source]Adenosine triphosphate contains one low-energy and two high-energy phosphate bonds:

Low energy : ΔG0' = -14,2 kJ mol-1
High energy : ΔG0' = -30.5 kJ mol-1
- ATP is regenerated from ADP (adenosine diphosphate), Pi and energy (from food); H2O is released in the process.
- ATP is the short-term energy "currency" of the cell.
- ATP concentration in the cell is low (ATP: 2-8mM; ADP:0,2-0,8mM). ATP is generated in high "turn over".
- ATP performs its chemical work through coupled reactions.
- Coupled reactions are always Ⓟ transfers, never direct hydrolysis
Basically, any ATP-driven reaction is reversible, building ATP from ADP and Pi in the process. However, some ATP-driven reactions should never be reversed; these include nucleotide and protein synthesis. If these were reversed, the organism would disassemble its own DNA and proteins for energy, a rather unfortunate strategy. For reactions that should never be reversed, ATP can be broken down into AMP (adenosine monophosphate) and PPi, which in turn becomes 2×Pi. This reaction has a ΔG0' of -65,7 kJ mol-1, which is totally irreversible under in vivo conditions.
It should be noted that AMP can not directly be converted to ATP again. Instead, the enzyme AMP kinase forms two ADP molecules from one ATP and one AMP. The resulting ADPs are then treated as described above.
Non-covalent bonds
[edit | edit source]The destruction of covalent bonds takes up huge amounts of energy. The breakdown of an O2 molecule into two oxygen atoms needs ~460 kJ mol-1. Thus, nowhere in "living" biochemistry are covalent bonds actually destroyed; if one is broken, another one is created. This is where non-covalent bonds come in, they are weak enough to be broken down easily, and to form "bonds" again. For this reason, many biochemical functions are using so-called weak/secondary/non-covalent bonds.
Weak bonds are created and destroyed much more easily than covalent ones. The typical range of energy needed to destroy such a weak bond is 4-30 kJ mol-1. Thus, the formation of weak bonds is energetically favorable, but these bonds are also easily broken by kinetic (thermal) energy (the normal movement of molecules). Biochemical interactions are often temporary (e.g., a substrate has to leave an enzyme quickly after being processed), for which the weakness of these bonds is essential. Also, biochemical specificity (e.g., enzyme-substrate-recognition) is achieved through weak bonds, utilizing two of their major properties:
- Since individual weak bonds are, well, weak, several of them have to occur in a specific pattern at the same time in roughly the same place.
- The short range of weak bonds.
The link that follows demonstrates the type of non-covalent forces: [1] There are three basic types of weak bonds, and a fourth "pseudo-bond":
Ionic bonds
[edit | edit source]Ionic bonds are electrostatic attractions between permanently charged groups. Ionic bonds are not directed. Example:
- X-CO2- ..... H3+N-Y
- ~ 20 kJ mol-1
Hydrogen bonds
[edit | edit source]Hydrogen bonds are also established by electrostatic attraction. These attractions do not occur between permanently charged groups, but rather between atoms temporarily charged by a dipole moment, resulting from the different electronegativity of atoms within a group. Hydrogen bonds are even weaker than ionic bonds, and they are highly directional, usually along a straight line. Besides being weaker than ionic bonds, hydrogen bonds are also weaker, and longer than similar covalent bonds. Hydrogen bonds are unique because they only exist when the Hydrogen is bonded to an oxygen (O), Nitrogen (N), or Fluorine (F), but the most common hydrogen bonds in biochemistry are:
- X-OH ..... O-Y
- X-OH ..... N-Y
- X-NH ..... O-Y
- X-NH ..... N-Y
Hydrogen bonds equal an energy between 12-29 kJ mol, whereas covalent bonds are much higher. For example, the covalent bond between oxygen and hydrogen is about 492 KJ mol-1.
Hydrogen Bonds and Water
[edit | edit source]Water has unique properties; after all, it is chosen to be the universal solvent. The unique properties of water are due to hydrogen bonding between all the oxygen and hydrogen atoms of the content. The hydrogen bonds occurring in water are about 2 angstroms apart from each other. Although hydrogen bonding is only about 5% as strong as covalent bond, they still cause water to have a high boiling point, and a high surface tension. The following link will take you to the structure of water and its Hydrogen Bonding.
Van der Waals attractions
[edit | edit source]Van der Waals attractions are established between electron density-induced dipoles. They form when the outer electron shells of two atoms almost (but not quite) touch. The distance of the atoms is very important for these weak interactions. If the atoms are too far apart, the interactions are too weak to establish; if the atoms are too close to each other, their electron shells will repel each other. Van der Waals attractions are highly unspecific; they can occur between virtually any two atoms. Their energy is between 4-8 kJ mol-1.
Hydrophobic interactions
[edit | edit source]Hydrophobic forces are not actually bonds, so this list has four items, but still just three bond types. In a way hydrophobic forces are the negation of the hydrogen bonds of a polar solute, usually water, enclosing a nonpolar molecule. For a polar solute like water, it is energetically unfavorable to "waste" a possible hydrogen bond by exposing it towards a nonpolar molecule. Thus, water will arrange itself around any nonpolar molecule in such a way that no hydrogen bonds point towards that molecule. This results in a higher order, compared to "freely" moving water, which leads to a lower entropy level and is thus energetically unfavorable. If there is more than one nonpolar molecule in the solute, it is favorable for the nonpolar molecules to aggregate in one place, reducing their surrounding, ordered "shell" of water to a minimal surface. Also, in large molecules, such as proteins, the hydrophobic (nonpolar) parts of the molecule will tend to turn towards the inside, while the polar parts will tend to turn towards the surface of the molecule.
References
[edit | edit source]Cooke, Rosa-lee. Properties of Water. Lecture 10. Mountain Empire Community College. n.d. Web. http://water.me.vccs.edu/courses/env211/lesson10_print.htm
Kimball, John W.. Hydrogen Bonds. Kimball’s Biology Pages. Feb. 12, 2011. Web. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/H/Hbonds_water.gif
Lower, Stephen. States of matter: Water and hydrogen bonding. General Chemistry Virtual Textbooks. 2009. Aug. 26, 2010. Web. http://www.chem1.com/acad/webtext/states/water.html
n.p. Covalent vs. Non-Covalent Bonds. n.d. http://www.pearsonhighered.com/mathews/ch02/c02cv.htm
W. W. Norton & Company. Hydrogen Bonding in Water. Web. 2012. http://www.wwnorton.com/college/chemistry/gilbert2/tutorials/chapter_10/water_h_bond/
WyzAnt Tutoring. WyzAnt Tutoring. Bonds. 2012. Web. http://www.wyzant.com/Help/Science/Chemistry/Bonds/

![{\displaystyle {pH}={pK}_{a}+log{[base] \over [acid]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c76e01f52dd2a8970a93ded61f409e95ebe9726a)