Biochemistry/Peptide Bond Formation
The biochemical mechanism of peptide bond formation is below. For a more chemical description, please consult the Organic Chemistry textbook.
Note: As with any biochemical mechanism, the following is enzyme-catalyzed and does not occur spontaneously in the cell. Enzyme active sites are left out for clarity.
Biochemical Mechanism
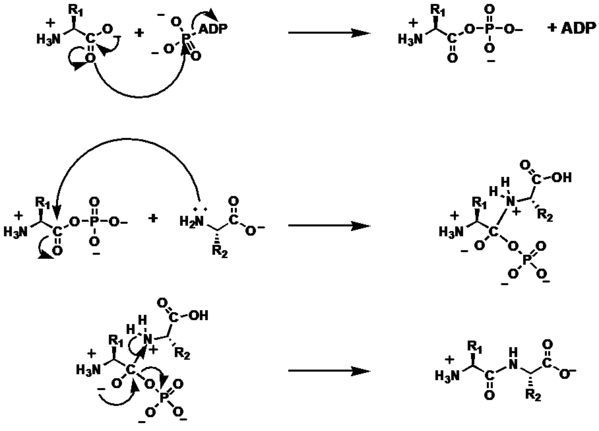
[edit | edit source]The addition of a the amino-terminus of an amino acid to the carboxy-terminus of a second amino acid goes through two primary steps:
- Carboxylate Activation
- Nucleophilic Addition/Formation of the tetrahedral intermediate
- Collapse of the tetrahedral intermediate
Carboxylate Activation
[edit | edit source]The carbonyl lone pair of the carboxy-terminal carbonyl attacks the terminal phosphate on a molecule of ATP via an SN2 reaction. The ADP acts as a leaving group, and the hydroxyl group on the carboxylate donates into the forming carbocation. Finally, the new carbonyl on the now phosphate ester is deprotonated.
Nucleophilic Addition
[edit | edit source]The lone pair of the amino-terminus on the second amino acid attacks the carbonyl carbon of the phosphate ester, forming a tetrahedral intermediate with four substituents on the former carbonyl carbon: the oxylate ion, the phosphate, the nucleophile, and the alpha carbon of the first amino acid. Finally, the amine nucleophile is deprotonated to a neutral state.
Collapse of the tetrahedral intermediate
[edit | edit source]A lone pair on the oxylate ion collapses on the phosphate carbon, and the inorganic phosphate is lost as a leaving group. The resulting bond is an amide bond between the carbonyl carbon and the amine group.