Biomechanics/The Biomechanics Of Skeletal Muscles
Let's begin now. This part has a lot of biology, but like the biology of biophysics in general, it's quite interesting. You won't be taught systematic or morphology or anything that actually doesn't have a link to some physical mechanism. So the biology here is not "pure biology" just for knowledge, which is really a relief for people like me.
Ok, now let's see what we've got. Try to use your imagination as some mechanisms need you to picture what happens in the three dimensions... When you are ready, begin.
Skeletal muscle morphology
[edit | edit source]
Connective tissues in muscles
- Epimysium
- Outer covering of muscles(fascia).
- Perimysium
- Internal connective sheath separating large portions of muscle tissue.
- Endomysium
- Internal connective sheath separating individual groups of muscle cells.
- Tendons
- Connective tissues connecting muscles to bones.
Muscle bundles and muscles fibers
- Muscle bundle (fasciculus)
- Group of muscle fibers enclosed by perimysium and subdivided by endomysium.
- Muscle fiber (cell)
- Multinucleate syncytium resulting from the fusion of many embryonic muscle cells (myoblasts).
Muscles Systems
[edit | edit source]a. Membrane system:
- Sarcolemma: plasma membrane of the muscle cell. It conducts action potential (AP). Motor endplates and many openings to the T-tubules are found on the sarcolemma.
- T-tubules (transverse tubules): system of tubules continuous with both sarcolemma and sarcoplasmic reticulum. They pass from one side of the cell to another and are filled with extracellular fluid.
- Sarcoplasmic reticulum (SR): the internal membrane system of the cell. It is connected to the T-tubules. When AP moves along the sarcolemma, it enters the T-tubules and sets off a general depolarization of SR.
- T-system: SR that is associated with the T-tubules.
- Cisternae: regions of SR that concentrate large amounts of Ca2 by actively removing it from the sarcoplasm. Thus, they contain
- Ca2 ATPase (a Ca2 pump).
- Voltage dependent Ca2 channels that can release Ca2 back into the sarcoplasm when the SR is depolarized.
b. Contractile system:
Myofibrils: bundles of 4 types of proteins. They produce the contractile force. There are 2 types: thick myofibrils and thin myofibrils.
c. Support system:
- Produces the ~P needed for contractions.
- Serves as a road for Ca2 to flow from cisternae to the thin fibrils.
- Supports general functions of the cell (protein synthesis…etc.).
- It consists of:
- Sarcoplasm: the cytosol. It is the seat of the glycolytic reactions to form ~P from carbohydrates. It thus contains stores of glycogen and ~P (in form of ATP and phosphagen).
- Mitochondria: located next to the fibrils. They are present in all muscle cells but vary in no. In muscles that depend heavily on O2 for ~P generation, they make up 50% of cell volume. In cells that are mainly anaerobic, they make up less than 1% of cell volume.
Muscle contraction
[edit | edit source]§ Muscle contraction depends on the hydrolysis of ~P from ATP.
§ The striations on muscle cells consist of alternating light and dark regions. (see image above)
a. Light I bands (isotropic):
Ø The middles of I bands have transverse lines called Z-lines.
Ø I bands are composed of thin filaments that
- Are fixed to the Z-lines.
- Are arranged in a lattice.
- Are made of 3 types of proteins: actin, troponin and tropomyosin.
- Have many glycolytic enzymes, indicating the importance of ATP produced.
Ø Sarcomere = area between 2 adjacent Z-lines.
b. Dark A bands (anisotropic):
Ø At rest, A bands have a light colored central region called H zone.
Ø H zone is made of thick filaments composed of 1 type of protein: myosin.
Ø A bands are composed of both thick and thin filaments, so they are the darkest.
c. During contraction:
Ø According to the “Sliding filament theory”,
1) The thin filaments move along the thick filaments and move further into the H zone, slowly obliterating it.
2) Since the thin filaments are fixed to the Z lines, the sarcomere decreases in length.
Ø The “Cross-bridge theory” replaced the former theory to explain the physical connections (cross-bridges) between the thick and thin filaments.
Studies used to explain muscle contraction
[edit | edit source]a. X-ray crystallography:
To see how proteins’ shapes (conformations) change with respect to different conditions.
b. Protein biochemistry:
To explain the chemical properties of proteins involved in contraction.
c. Histochemistry:
To test ideas about muscle contraction on real cells. The techniques include injection of dyes to change color according to Ca2 present in the sarcoplasm or according to voltage.
The thin filaments
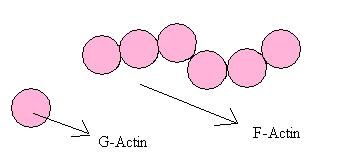
[edit | edit source](Please look at the thumbnail picture at the beginning for further understanding).
a. Actin:
Ø Most of the thin filament is a double helix of 2 F actin polymers.
Ø Each F actin is made of a large no. of single actin molecules called G actin (globular proteins, MW = 45000).
Ø Each G actin can bind to
- Other G actins to form a polymer.
- Troponin.
- Myosin.
b. Troponin:
Ø Least common protein.
Ø It is the filament-level regulator of crossbridge formation.
Ø It has a quantenary structure made of 3 subunits:
1) Troponin-I binds actin to troponin-C.
2) Troponin-C (most important), can bind up to 4 Ca2 .
3) Troponin-T binds troponin-C to tropomyosin.
Ø It is allosteric: it can change shape in response to the amount of Ca2 that has bound to it.
Ø The steep part of the curve indicates that a slight change in Ca2 causes a large effect on troponin’s shape.
c. Tropomyosin:
Ø Rod-shaped molecule attached to troponin.
Ø At rest, tropomyosin is held over the myosin binding sites on the actin.
Ø During contraction, Ca2 is high and tropomyosin is moved away from the binding sites to let myosin bind to actin. This occurs by the binding of troponin to 4 Ca, then it deforms and “bends”. Doing so, it moves the tropomyosin in the groove between the 2 actin filaments, letting the actin binding site to myosin revealed.
Ø So, the role of tropomyosin is to produce “steric hindrance” to the binding of myosin to actin.
d. Polarity:
The thin filaments show a polarity: they only bind with myosin heads that point in a certain direction. This polarity reverses at each side of the Z-line.
The thick filaments
[edit | edit source]§ They are composed of myosin, a very large (MW = 470000), quantenary (6 chains) allosteric protein.
§ Each myosin consists of 2 identical subunits wrapped around each other. Each subunit consists of 1 heavy chain and 2 different light chains.
a. Components of the heavy chain:
1) Tail: long, very insoluble units that polymerize with each other to form what is viewed as the thick filament.
2) Neck: its material is similar to that making up the tails, except that it is more water-soluble and is flexible.
3) Globular heads: large and water-soluble. They contain
o Binding sites for actin. So, together with necks, they make the cross-bridges.
o ATPase.
b. Light chains:
They are attached to the globular heads of the heavy chains. Have regulatory function
c. Polarity:
1) Polarity results from the way the myosin tails are connected together.
2) Myosin polarity reverses at the center of each sarcomere.
3) All myosin heads on one side of the center move together, pulling the thin filament towards the center of the sarcomere.
d. Note:
§ Each thick and thin filament is organized in a 3D lattice that permits each filament to form cross-bridges with several others.
§ Contraction occurs by the heads of myosin, pulling the actin filaments towards the center of the sarcomere. This "pulling" by heads is done by continuously breaking and building a bond between the head and the neck of myosin (imagine a bond between your thumb and your index. As the bond breaks and rebuilds, your thumb continuously goes and move away from your index. This "leaving-returning" mechanism allows the myosin to pull the actin).