Chemistry Friends/Printable version
| This is the print version of Chemistry Friends You won't see this message or any elements not part of the book's content when you print or preview this page. |
The current, editable version of this book is available in Wikibooks, the open-content textbooks collection, at
https://en.wikibooks.org/wiki/Chemistry_Friends
Scientific method applied to Chemistry
Empiricism
[edit | edit source]empirical descriptions
[edit | edit source]tables of independent and dependent variables
[edit | edit source]graphs
[edit | edit source]empirical hypotheses
[edit | edit source]empirical definitions
[edit | edit source]generalizations
[edit | edit source]scientific laws
[edit | edit source]Theory
[edit | edit source]theoretical descriptions
[edit | edit source]theoretical hypotheses
[edit | edit source]theoretical definitions
[edit | edit source]Standards
[edit | edit source]STP
[edit | edit source]STP is an acronym for "Standard Temperature and Pressure". STP is 100 degrees Celcius, or 373 degrees Kalvin, and 101.3 kPa.
SATP
[edit | edit source]SATP is an acronym for " Standard Ambient Temperature and Pressure", which is, simply put, room temperature. It is 25 degrees Celsius (298 degrees Kalvin), and 100 kPa.
Matter
Separating Matter
[edit | edit source]mechanical
[edit | edit source]To separate mechanically, matter is divided manually or with a magnet([1]p.38).
settling
[edit | edit source]Some mixtures can be separated by letting it sit so one substance sinks to the bottom of the container. This method is normally very slow but can be sped up by spinning the container ([1]p.39).
floatation
[edit | edit source]Air is blown into the compound, causing some parts of it to froth and float to the top. The top portion is then skimmed off ([1]p.38).
filtration
[edit | edit source]If you have a heterogeneous mixture with solids mixed in it, you can pour it through a filter to separate the solids from the fluids ([1]p.38).

extraction
[edit | edit source]You can mix something into your solution that is soluble with some of the elements in the solution but not all of them ([1]p.39).
fractional distillation
[edit | edit source]Fractional distillation is achieved by boiling a liquid compound at different temperatures. The components vaporize at separate temperatures and so they can be separated ([1]p.39).

crystallization
[edit | edit source]In a compound where a solid is dissolved in a liquid is cooled to form crystals of the solid element. This happens naturally with salt or sugar water ([1]p.39).
chromatography
[edit | edit source]The mixture is dissolved into a solvent. The solvent goes through a porous material such as filter paper, and the elements of the compound separate at different rates in porous material. This creates rings or lines of different elements ([1]p.39).
classifying matter
[edit | edit source]pure substances
[edit | edit source]elements
[edit | edit source]Everything is made up of elements. We are mostly carbon, hydrogen, oxygen, nitrogen, calcium and phosphorus[2]. Elements consist of: protons, electrons, and neutrons. Neutrons and protons stay in the nucleus while the electrons orbit around.
compounds
[edit | edit source]When two or more elements are combined with definite proportions they are known as compounds [3]. Water (H2O) and salt (NaCl) are both compounds because they are made of more than one element.
mixtures
[edit | edit source]homogeneous mixtures
[edit | edit source]In a homogeneous mixture, you can't see the separate components. The prefix "homo" is used, because it indicates sameness. Homogeneous mixtures are often called solutions, or when the particles are slightly bigger, colloids. The particles in a homogeneous mixture are invisible to the naked eye, except for certain colloids where the particles may be seen if in a direct shaft of light [4].Metals such as stainless steel and bronze are homogenous mixtures.
heterogeneous mixtures
[edit | edit source]Heterogeneous mixtures are mixtures where the different components are visible to the naked eye, "hetero" indicating difference. Suspensions are heterogeneous mixture that, when allowed to sit, will separate into their different components. Mixed vegetables, beach sand and oil-vinegar mixtures are all examples of heterogeneous mixtures [4].
Elements
[edit | edit source]Classifying elements
[edit | edit source]There are a few ways to write the names for molecules. Every element on the periodic table has a name and a symbol. For instance, you can write the element hydrogen in two ways. Either you can just write out the name hydrogen, or you can write its symbol,H. The symbol is one or two (and in some cases, three) letters. The first letter is capitalize and the next is small.
Naming Ionic Compounds
[edit | edit source]When elements stick together in an ionic compound, there is a special way to write the compound. Say the is a compound of zinc and oxygen. Its names would be 'Zinc oxide' when writing the name, we change the last syllable of the last element to -ide. When you write the chemical formula, you use the symbols instead of the whole word. Also, you need to specify how many zinc and oxygen atoms are in each compound. Zinc has a charge of 2+ while oxygen is 1-. In order to make a stable compound, you want the charges to add up to zero. So you'd need two oxygen atoms for every zinc. This is the chemical formula: ZnO2. When naming an ionic compound with its full names, you don't need to say the amount to each element but you do if you are writing the symbols.
Naming Molecular Compounds
[edit | edit source]Naming molecular compounds is very similar to naming the Ionic ones. However, when naming the compound you need to specify how many of each atom there are. For instance, water's chemical formula is H2O. We write its full name dihydrogen oxide. we put the prefix 'di' on the hydrogen to say that there are two hydrogen atoms on each oxygen. Here's a list of the prefixes 1-10 ([1] pg.113)
- mono (you don't really need to write this, but you can.)
- di
- tri
- tetra
- penta
- hexa
- hepta
- octa
- ennea
- deca
Periodic table
[edit | edit source]periodic law
[edit | edit source]The periodic law is a law about the arrangement of elements according to their mass discovered by John Alexander Newlands in 1864. It stated: "When elements are arranged in order of increasing atomic mass, chemical and physical properties form patters that repeat themselves at regular intervals". ([1]p.63).
family/group
[edit | edit source]The periodic table is divided into eighteen different columns, known as "families" or "groups". Each family of elements have similar characteristics, such as the number of electrons in their outer orbital or the way they react with other elements. The first family, which includes Sodium and Francium, have only one valence electron and all react violently with water.
alkali metals
[edit | edit source]Alkali metals are the metals in the very first group on the periodic table, on the far left side, excluding hydrogen, which is merely in that row for organizational purposes. Every element in this family have an ion charge of 1+, and have only one valence electron. This means that they are highly unstable and react very quickly with other elements. Alkali metals are especially reactive with water. The higher their atomic number, the more violently they react because they are more unstable. Rubidium and Potassium are both alkali metals.
alkaline-earth metals
[edit | edit source]Alkaline Earth metals are the elements in the second family, near they left side of the table. They have an ion charge of 2+ and they are quite reactive.
transition metals
[edit | edit source]All the metals from the third group to the staircase line are known as "transition metals". They have varying ion charges, with some of them having more than one, and they have varying levels of reactivity. This section of elements contain many of the metals that we use daily, such as iron, copper, and tin.
halogens
[edit | edit source]Halogens are the non-metals located on the second last family of the periodic table, near the far right side of the table. They are highly reactive elements because of their ion charge of 1-.
noble gases
[edit | edit source]Noble gases are the gases in the family to the far right side of the periodic table. They are known as "noble" because they are the only elements with a full valence shell, and as such they will not react with other elements easily. They are elements that are stable in normal conditions. These elements light up in different colours when an electric charge is passed through them. Helium, Neon and Argon are all noble gases.
lanthanides
[edit | edit source]The series of elements that starts after Lanthanum is called Lanthanides
actinides/transuranic
[edit | edit source]The series of elements that start after Actinium is called Actinides.
staircase line
[edit | edit source]This is the line between the metals and the nonmetals. It is called the staircase line because it resembles the steps of a staircase,
Metals
[edit | edit source]Metals are found on the left side of the Periodic Table.
![The elements that are coloured light blue are the noble gases, the yellow ones are halogens, they are both part of a group of nonmetal, which are coloured green. The metalloids are coloured brown. The post-transition metals are periwinkle. The pink metals in the main body of the table are transition metals, the top row of elements on the section under the main periodic table are the lanthanids, underneath them are the actinides, the Alkaline are the peach ones, and the Alkali metals are the red ones[5].](http://upload.wikimedia.org/wikipedia/commons/thumb/8/84/Periodic_table.svg/779px-Periodic_table.svg.png)
Nonmetals
[edit | edit source]These are found on the right side of the staircase line.
Atomic theory
[edit | edit source]Early Greek Theories
[edit | edit source]The early Greeks only had the technology available to identify a few elements. The first seven elements identified were gold, silver, iron, mercury, tin, copper and lead. At the time, the Greeks only knew of seven planets or celestial bodies, and so gave each of the elements a celestial body and a symbol to represent them. The Greeks knew very little about the chemical properties of the elements, they merely used them for making tools and art. As time went on, it became apparent that having symbols would not be sufficient for all the elements, and so it was eventually changed to letters. ([1]p.55).
Dalton's Atomic Theory
[edit | edit source]Dalton's theory is often called the billiard ball theory. He believed that atoms were neutrally charged spheres, with no real distinction between protons and electrons.
Bohr's Theory
[edit | edit source]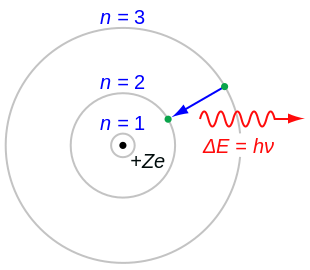 Bohr was the first chemist who theorized that elements have a positively charged nuclues with negatively charged electrons orbiting around it, where the electrons were divided into orbital groups, with a specific number of electrons in each shell. He figured that there would be a certain number of electrons in each orbital, and the number of electrons in the last shell would determine the chemical properties.
Bohr was the first chemist who theorized that elements have a positively charged nuclues with negatively charged electrons orbiting around it, where the electrons were divided into orbital groups, with a specific number of electrons in each shell. He figured that there would be a certain number of electrons in each orbital, and the number of electrons in the last shell would determine the chemical properties.
Electrons
[edit | edit source]Electrons are negatively charged. They orbit around the nucleus. Each orbital requires a certain amount of electrons. When there aren't enough electrons to fill the orbital, the atom is positive. When the opposite happens and the atom has to open up a whole new orbital just for one or two little electrons. This atom is negatively charged. When a positively charged atom such as a sodium finds a negatively charged atom like chlorine, the chlorine gives his extra electron to the sodium who needs an extra one. Then the two of them become an ionic compound, sodium chloride.
Nucleus
[edit | edit source]The nucleus is the center of an atom. It comprises of both the positively charged protons and neutrally charged neutrons. It is the nucleus of the atom that makes up most of the weight of the atom, because electrons do not weigh enough to be significant. Each proton or neutron weighs 1 AU (Atomic Unit). You can find out how many protons or neutrons there are in an element by looking at the molar mass and the atomic number. The atomic number will be the number of protons, and the atomic number subtracted from the molar mass will be the number of neutrons.
Atomic Number
[edit | edit source]The atomic number is the number of the element on the periodic table. It signifies how many electrons are orbiting the nucleus of the element, so as the atomic number gets higher, so does the number of electrons. For example, Carbon's atomic number is 12. That means it has 12 electrons, and therefore 12 protons. Elements are grouped together based on their atomic numbers, because the number of electrons an element has can affect it's properties.
Neutrons
[edit | edit source]Neutrons are the sub-atomic particle that has a neutral charge. They are located in the nucleus of the atom and weigh 1 AU. The number of neutrons in an element determine if it is an isotope or not.
Isotope
[edit | edit source]An Isotope is an element with the normal number of protons and electrons, but has extra or fewer neutrons. The element is still neutrally charged because the protons and the electrons are still balancing each other out. The extra or missing neutrons add or subtract weight to the element. Isotopes can be found in nature, but they are generally not as stable as the normal version of the element. ([1]p.76).
Mass Number
[edit | edit source]The mass number is the molar mass of an atom. It comprises of the combined mass of both the protons and the neutrons of the element. On the periodic table, the molar mass will rarely be a round number. This is because it is the average mass of all common isotopes of the element. Hydrogen has very few common isotopes, so it's atomic number is 1.01.
Quantum Mechanics
[edit | edit source]Monoatomic Ions
[edit | edit source]Monoatomic Ions are elements that have either gained or lost electrons to fill their valence shell. This makes them have either a positive or negative charge. They are also known as ions [1].
cations
[edit | edit source]Cations are elements that have lost electrons to fill their valence shell. They have a positive charge as an ion because they have more protons than they do electrons. All alkali metals are cations because they will lose electrons rather than gain them to fill their last shell.
anions
[edit | edit source]Anions are the opposite of cations in that they have a negative charge because they have gained electrons to fill their valence shell. Most non-metals will be anions because they are near the right side of the periodic table and will gain electrons rather than lose them. A goofy trick to remember which is which is to visualize a happy cat. This will help you remember that cations are positively charged.
Quarks
[edit | edit source]Quiz
[edit | edit source]Here is a short quiz on the Matter section:
References
[edit | edit source]- ↑ a b c d e f g h i j k l m Jenkins, F., van Kessel, H., Tompkins, D., Lantz, O., (1996). Nelson Chemistry, British Columbia, Nelson Canada.
- ↑ Chem4Kids[1997-2012]http://www.chem4kids.com/files/elem_intro.html
- ↑ Chemistry and You! (n.d.) [1]
- ↑ a b Ophardt, C., Virtual Chembook,(2003), [2]
- ↑ Dayah, M. (1997) Dynamic Periodic Table, http://www.ptable.com
Chemical Bonding
Ionic bonds
[edit | edit source]An Ionic bond is a bond where one element give away electrons to another element. This makes one element positively charged and the other negatively charged. Ions are elements who have gotten rid of or gained electrons. For instance, sodium has one electron in its valiant cell. This electron is very inconvenient so the sodium just gets rid of the extra electron. Then, sodium has 11 protons and 10 elections. This makes sodium 1+.
polyatomic ions
[edit | edit source]These are ions that work together like one ion. If you had NaNO3, The Na would have bonded with NO3 and not three Os and an N. This is because these elements work together lie one ion.
Ions of Multi-Valiant Metals
[edit | edit source]When working with an ion that can have different numbers of protons, the valences need to be taken into account. If you have an iron oxide compound, it could either be FeO or Fe2O3. When the iron has a charge of 2+, then it bonds with the oxygen (which has a charge of 2-) with one of each. This makes FeO. If the iron has a charge of 3+, then it has to from with the oxygen with two irons and three oxygens. Thus creating Fe2O3.
Covalent bonds
[edit | edit source]Covalent bonds are elements that borrow electrons. Oxygen needs two more electrons to fill his valiant shell. When he bonds with another oxygen he solves this problem. The first oxygen borrows two of the second oxygen's electrons some of the time and the second oxygen uses two of the first oxygen's electrons. This isn't as strong of a bond as an ionic bond.
polar covalent bonds
[edit | edit source]When oxygen forms with two hydrogens, it forms water. Oxygen is more negative than hydrogen. Since opposites attract and things with the same charge repel each other, the hydrogens are on opposite sides of the oxygen. The different poles on a covalent bond make it interact differently with other compounds like itself. When water is in liquid form, the molecules slip by without too much difficulty. The opposites of different compounds do attract a bit, but nothing crazy happens. However, when the compound freezes, something crazy happens! Usually elements bunch up together and become more dense than the liquid, but that is not the case with polar covalent bonds. The elements with the same charge refuse to be bunched together. Instead, they form this large, intricate matrix where the opposites touch but nothing else does. There is lots of empty space in between the elements so the solid is much less dense than the liquid. That is why ice floats in water and iron sinks in molten iron.
Gases
Properties and Patterns
[edit | edit source]When elements are in their gaseous form, they follow the following rules.[1]
- They fill their container but don't have a volume of their own.
- They are very compressible and can be pushed into a space much smaller than usual.
- They mix through the air without being mixed
- When they get hotter they expand
Pressure and Volume
[edit | edit source]Pressure is force per unit of area. If you had a book with a large area and pushed it softly, it might have the same amount of pressure as a pin if you pushed it really hard. The SI unit for pressure is the kilopascal. One kilopascal (kPa) is equal to 1000 newons of force over 1 square meter. Or 1kPa=1000N/1m2. We experience pressure every day. When we lay in our bed the blanket pushes us down. When we sit down for breakfast, we up pressure on our chairs. Even the air had pressure. This is called 'atmospheric pressure'. The average Atmospheric Pressure at sea level is 101 kPa. We call this (101 kPa) 'one standard atmosphere' or 'atm'. When the conditions are all normal and the temperature is 0°C and the atmospheric pressure is at atm, we call it 'standard temperate and pressure' or 'STP'. When the pressure is 100 kPa and the temperature is 25°C it is called 'standard ambient temperature and pressure" or 'SATP'. Just remember that STP has less letters and less heat while SATP has more letters and more heat.[1]
Boyle's law
[edit | edit source]Robert Boyle was an English chemist. He developed the law about pressure and volume. He realized that if you compress a container of gas the volume will decrees with the same proportions. If you doubled the pressure, the volume would be halved. This is the equation. [1]
- v1p1=v2p2
The first side of the equation is how things where initially, (v=Volume and p=Pressure) the second side is how the Volume and pressure were after they changed.
Temperature and Volume
[edit | edit source]Jacques Charles was a French physicist. He noticed a relationship between the temperature of a gas and its volume. He noticed that as the temperature of a gas increased, the volume increased proportionately. This can be shown in a few different equations.[1]
- v=kT
The v represents volume, the T is temperature and k is a constant value. We can also write the equation like this.
- v1/T1=k and this v2/T2=k
Or it can be simply written together like this:
- v1/T1=v2/T2
The Combined Gas Law
[edit | edit source]Charles and Boyle both made useful formulas to calculate the changes in gasses. If we combine their theories, we can make a very useful formula that can help us calculate anything we need to know about gasses. The formula looks like this.[1]
p1v1/T1=p2v2/T2.
The letters stand for volume (v), pressure (p) and temperature (T). The nice thing about this equation is that we can customize it to fit our question. If the pressure remains constant we can take it out of the equation and simply write:
p1v1=p2v2
Kelvin Temperature Scale
[edit | edit source]Scientists use the Kelvin Scale when measuring temperature. It is written in Kelvin and not degrees Kelvin. When converting Celsius to Kelvin, add 273°C. So if we wanted to know how hot it was on a day in the summer, we'd add 273°C to 25°C. That day, it would be 298 K outside! When writing in Kelvin, the temperature is always a positive number. Not even insanely cold temperatures are negative. This is because 0 Kelvin is Absolute Zero. No where in the universe are things that cold or colder. Absolute Zero means the particles themselves have stopped moving. Even in space the stars keep things from getting that cold.[1]
Avogadro's Theory and Molar Volume
[edit | edit source]Before a theory is accepted, experiments have to be done to prove it. If an idea helps explain lots of unknown phenomena, it is even better.[1] If you walked into you house and saw your grandmother's shoes on the shoe rack and heard her voice coming from the living room, you may come up with a few theories. One, your grandmother came to visit or two your grandmother left her shoes on the shoe rack when she was sneaking through your house last night and the voice in the living room is a parrot that can mimic voices. You would probably got with theory one: If you conclude that your grandmother is over it explicates two things. Also you may have observed that your grandmother drops by a lot in the afternoons. Theory two makes less scene. You have never known your grandmother to be a forgetful person or go any where at night. Also, you don't own a parrot. If a theory explains lots of things, then it usually makes more sense. The kinetic molecular theory was accepted amoung scientists for this reason. It explains why gases are compressible and why there's gas pressure. It also complements Boyle's and Charles's laws.[1]
The Law of Combining Volumes
[edit | edit source]Joseph Gay-Lussac was a french scientist. He discovered the Law of Combining Volumes. The law says that if you combine gases the ratio of molecules is in simple whole numbers.[1]
Avogadro's Theory
[edit | edit source]Avogadro found the reason for the simple ratios. His theory states that if you two containers containing equal amounts of gas at the same temperature, they would have the same amount of molecules.[1] (I don't know how he proved this but I doubt he counted them all.)
The Law of Combining Gas Volumes
[edit | edit source]Because of the Law of Combining Volumes and Avogadro Theory, we have a simple way of figuring out the amount of each molecule in an equation.[1] Say we knew that hydrogen and oxygen make dihydrogen oxide or in other words:
- O2 + H → H2O
According to the Law of Combining Volumes, the number of dihydrogen oxide molecules should be a simple whole number. Right now, the equation is wrong. It says two oxygen atoms plus one hydrogen atom equals two hydrogen atoms and one oxygen atom. We still need to balance the equation. Let's start with oxygen. There is one oxygen atom needed in dihydrogen oxide but it is in groups of two on the left side of the equation. Therefore, this equation makes two molecules of dihydrogen oxide. Now for the hydrogen. Since we've decided that there are two molecules of H2O, that means there are four hydrogen atoms needed. So there are four atoms of it on the left side of the equation. It will look like this:
- O2 + 4H → 2H2O
Molar Volume of Gases
[edit | edit source]A mole is a number of particles. It's a bit like saying a dozen but it's a more complicated number. The molar volume of a gas is one mole of the gas at a specific temperature. This volume doesn't change between gases. A gas at STP has a molar volume of 22.4 L/mol. Scientists like to use moles a lot. Working with volume is easier than mass. It is also more exact. You can use molar volume to convert between moles and liters.
Molar Volume and Molar Mass
[edit | edit source]The Ideal Gas Law
[edit | edit source]References
[edit | edit source]
Solutions
Classifying Solutions
[edit | edit source]A solution is when two or more things mix together homogeneously. These things are called the solvent and the solute. Solutes can be solid, liquid or gas. One of the most popular solvent is water.[1]
Solutions of Electrolytes and Non-Electrolytes
[edit | edit source]An electrolyte is an aqueous solution that conducts electricity. Pure water isn't an electrolyte, but if you dissolve something in water, it can conduct electricity. Acids and bases are electrolytes. Molecular compounds usually aren't.[1]
Acidic, Basic, and Neutral Solutions
[edit | edit source]Solutions can be acidic, basic, or neutral. The stronger the acid, the more elecricly conductive it is.[1]
Understanding Solutions
[edit | edit source]When something is dissolved into a solution, the compound dissociates. This means each molecule and ion goes off in its own with water. If you put NaCl in water, in would dissociate and become Na+ and Cl-. The sodium chloride wouldn't be separated, the ions would just get farther apart. Dissociation makes solutions electrolytes. The Na is positive and the Cl is negative, so if they are dissociated in water, the charges help electricity flow through the solution. This works with acids and bases too. If an acid or base dissolves, it dissociates. Then one of the ions is negative and one is positive.[1]
Substances in Water
[edit | edit source]Some things dissolve in water better than others. Ions' solubility is very predictable. There are tables to show how soluble different ions are. Molecules are trickier. There is no simple was to predict how soluble different molecules are. You just have to remember.[1]
Reactions in Solutions
[edit | edit source]Chemicals are put into solutions to make it easier to transport, load and store them. It also helps with reactions. If something is in a solution, it can have a faster, more complete reaction and can change the results. Reactions are faster and more complete for this reason: a dissolved chemical has more surface area than if it were on its own. [1]
Net Ionic Equations
[edit | edit source]Let's say you have sodium oxide and chlorine and you mix it into a solution of sugar water. The equation might look like this.
- Na2O(aq)+C6H12O6(aq)+ Cl(aq)= NaCl + C6H12O6(aq) + O(aq)
Now we put the theory of dissociation into effect.
- Na2(aq)+O(aq)+ C6(aq) +H12(aq) +O6(aq) +Cl(aq) =NaCl + C6H12O6(aq) + O(aq)
The thing is, this equation seems pretty cluttered and some of the ions don't even seem to change. Let's cancel them out!
- Na2(aq)+
O(aq)+C6(aq) +H12(aq) +O6(aq)+Cl(aq) =NaCl +C6H12O6(aq)+O(aq)
This leaves us with the equation
- Na(aq)+ Cl(aq)= NaCl(aq)
That makes more sense![1]
Qualitative Chemical Analysis
[edit | edit source]There are two kinds of measurements: qualitative and quantitative. Qualitative is looking at litmus paper to see if it is blue or red, noticing bubbles in a solution and seeing rust is forming. You can see results but there are no units or numbers you can put in. That's what quantitative measurements are. A pH of three, twenty seven grams of HCl and two moles of oxygen are all quantitative. They are more exact than qualitative measurements.[1]
Qualitative Analysis by Colour
[edit | edit source]Some substances can be identified by colour. The colour of the solution might change, there might be a coloured gas or a flame. Litmus paper changes colour based on if the solution it is placed in is acidic or basic. When burned, copper(II) has a green flame.[1]
Concentration of a Solution
[edit | edit source]If a solution has very little solute in it, it is a dilute. If a substance has lots of solute in it, it is a concentrated solution.[1]
Communicating Concentration Ratios
[edit | edit source]Use this ratio to find the concentration of a solution.
- concentration=quantity of solute /quantity of solution
If there are 2 mL for every 100mL of solution, the ratio would be:
- 2mL/100mL and the answer would be 2% v/v.
The v/v thing means volume to volume. Some times you need to calculate w/v which is weight to volume. This would be like if there were 4 grams of sugar in a 100mL sugar water solution. There are ways to express very small units. Parts per million is one. If there was three grams of salt in a million mL lake, the concentration of salt would be 3ppm. There is a ratio to find the molar concentration. It is the amount of the solute in moles when dissolved in a liter of water.
- Molar concentration= amount of solute (in moles)/ volume of solution (in liters)
Molar concentration is measured in (mol/L)
Calculations Involving Concentration
[edit | edit source]In chemistry, it is nice to know the quantity, volume and concentration of a solution. If you only know two of these things, you can use a formula to figure the thrid one out.[1]
- mass/volume=concentration
Preparation of Standard Solution from a Solid
[edit | edit source]Standard solutions are solutions with a precise concentration.[1]
Preparation of Standard Solution by Dilution
[edit | edit source]Dilution is a way of changing the concentration of a solution by adding more of the solvent or some other liquid. If your lemonade is too strong, you might try adding water. This would be diluting the lemonade. Dilution is a pretty easy was to alter the concentration of a solution. Diluted solutions are used a lot today. If a chemical reaction happens too fast, a chemist can dilute the chemical and slow down the reaction. If a doctor needs to prescribe a thousandth of a mL of medicine to you, he might instruct you to take a mL of the medicine, mix it with a L of water and take a mL of the solution. It is easier to measure in milliliters and liters than it is to measure in thousandths of a milliliter. There are many tools to make exact measurements. Volumetric flasks like graduated cylinders and beakers and scales are very common tools. Another tool is called a volumetric. It is a long tube that looks like an eye dropper except the measurements on the side have 0 on the top and the biggest number on the bottom. To use, you fills the tube all the way to the zero and moves the volumetric over the container you want the measured solution in. Since the solution reaches the 0 mark, no solution has dropped into the container. Let's say you need three units of solution. Jiggle the nob on the top of the tube. This lets little bits of air into the tube so the solution drips out the bottom. Carry on until the solution line drops to the three. Put the volumetric away and you have 3 units of solution![1]
Calculating Dilution
[edit | edit source]It isn't too hard to calculate a concentration of a solution after it has been diluted. If the amount of solvent doubles, the concentration halves. There is a simple formula to figure it out.
- vici=vfcf
The vi is the volume before you diluted the solution. vf is the volume after the solution was diluted. c is for the concentration. i for initial and f for final. As long as you know three of the pieces of information, you can use the equation to find out the fourth one.[1]
Solubility
[edit | edit source]Every solution has a limit to how much of a solute can be added. If more than the limit is added, the access won't dissolve. It'll just sit there.[1]
Solubility Rules and Examples
[edit | edit source]When mixing substances, there are rules the solutions follow. They are nice to know so you can dissolve things better.
- If you heat water to a higher temperature, more solids solute can dissolve into it.
- If you want to get more gas to dissolve in the water, cool it down. Gas always dissolves better in cold water.
- Gases also dissolve better at high pressure.
- Some oils won't ever dissolve in water because they are immiscible. It's pretty much imposable so don't bother trying.
- The opposite to some thing that is immiscible is something that is miscible! This means there is no limit to how much of the substance you can dissolve. Well, the limit is how much of the substance you can get your hands on.
- Most elements don't dissolve well in water, but halogens and oxygen do alright. Also, metals from group I and II can dissolve pretty well.
You can see evidence of solutions all around. If you let the water evaporate from the solution, any solid solutes left behind form crystals. Stalagmites and stalactites are good examples of left behind minerals. You might also notice that when you open a can if pop, some air escapes. What that is is carbon dioxide that use to be dissolved in the drink when it was cold, but undissolved as the pop warmed up. Fish survive because of the oxygen dissolved in the water.[1]
Concentration of Ions
[edit | edit source]Moles of Ions and Ionic Compounds
[edit | edit source]In chemistry, it can be very useful to know the molar concentration of ions. First, let's remember that a mole is a number, like a dozen. It isn't an amount, it is a number. A complicated number, but a number none the less. So if we have one mole of an ion like CaCl2, There is one mole of CaCl2, one mole of Ca, and 2 moles of Cl. Just like if there were a dozen cats, there would be a dozen tails, two dozen eyes and four dozen paws. (Unless, of coarse, there were any mutant cats with three eyes or unfortunate cats who lost an eye.)[1]
Molar Concentration
[edit | edit source]So anyway, another thing we need to know is the symbol for molar concentration. It is square brackets. So [NaCl] is the molar concentration of sodium chloride.[1]
Calculating Molar Concentration
[edit | edit source]When we calculate molar concentration, we dissociate the ions and calculate them separately. So let's say we are looking for the molar concentration of CaCl2 in a 0.123 mol/L solution. First thing we do is dissociate the ions.
- CaCl2(aq) → Ca(aq) + 2Cl(aq)
Let's find the molar concentration of Ca first! We multiply the concentration by how many moles there are. In this case, there is one mole.
- [Ca(aq)] = 0.123 mol/L × 1 = 0.123 mol/L
So, There are 0.123 mol/L of carbon in the solution. Now for the chlorine. Remember, there are 2 moles of chlorine.
- [Cl(aq)] = 0.123 mol/L × 2 = 0.246 mol/L
There are 0.246 mol/L of chlorine![1]
Molar Concentration of Hydrogen
[edit | edit source]It is very important to know the molar concentration of hydrogen ions because hydrogen ions make acids. The higher the hydrogen concentration, the more acidic the solution. Pure water is neutral. It has a pH of 7. What this means is the hydrogen concentration of water is 1×10-7 mol/L. Something more acidic, like vinegar, has a pH of about 2 and therefore a hydrogen concentration of 1×10-2. Something basic with a pH of 11 would have a hydrogen concentration of 1×10-11.[1]
References
[edit | edit source]
Acids and Bases
Acids and Bases
[edit | edit source]Acids and bases are compounds that one or more "loose" hydrogens (H) or hydroxides (OH) attached to them. These hydrogens or hydroxides do not have a very strong bond to the compound, and they will break free very easily. It is the hydrogens and hydroxides, that give acids and bases their properties.[1]
How Our View of Acids and Bases Changed
[edit | edit source]People have noticed differences between acids and bases for ages. Differences between the two had been noticed since the middle ages. Discoveries about the pH scale and acid-base reactions began in the early 1900s.
Most of the early theories about acids and bases weren’t right but at least they were trying. In the mid-seventeen hundreds, a guy named Antoine Lavoisier came up with a theory. He concluded that oxygen and water made things acidic. Sulfuric acid was H2SO4 which could be made of H2O and SO3. So He thought it oxygen made it acidic. This wasn’t the case. Some solutions with oxygen in them, like CaO and are basic. There are also acids without oxygen in them.[1]
A guy named Sir Humphrey Davy concluded that Hydrogen was responsible for acidity. Another guy by the name of Justus von Liebig agreed with Humphrey and elaborated the theory. He hypothesized that hydrogen can form ionic bonds were the hydrogen act like the metal. He decided that acids were the salt of hydrogen. This was a good theory, but it didn't explain why loads of compounds with hydrogen in them are neutral, like water or basic, like NH3.
At last in 1887, Svante Arrhenius came up with a pretty good explanation. He concluded that acids were ionized in an aqueous solution. In the solution, they formed bond with the hydrogen ions. Bases did the same thing, except they formed ionic bonds with hydroxide. This might have been a little off, but it did explain somethings, like water being neutral. Because H2O is a combination of Hydrogen (H+) and hydroxide (OH-), it is neutral.[1]
Revising Arrhenius' Definition
[edit | edit source]Arrhenius was right about acids dropping hydrogen ions and bases picking up hydrogen ions but that theory wasn't complete. When acids drop hydrogen, it doesn't stay a lone hydrogen for very long, if there is any water nearby, the positive hydrogen ion will be attracted to the slightly negative side of the oxygen atom and forms H3O+. This is not a covalent bond. The hydrogen is just attracted to the slightly negative oxygen.[1]
Hydrolysis of Amphoteric Ions
[edit | edit source]Amphoteric ions are things that alone are not acidic or basic but, it will become so if you just add water. Take NaHCO3 for example. Alone, it doesn't look too acidic but, if you mix it in water, the sodium will disconnect. It's still close to the rest of the ions, still technically bonded to the HCO3. It's kind of like at a dance, if your not holding hands with your dance partner, your just close by. Then, something happens in the solution. The water wants to bond with the HCO3 so they just leave the Na there. It would be like if one of the dancers found someone else to dance with and just left. Really rude. Anyway, the HCO3 and the H2O bond, forming OH- and H2CO. The OH- is of course, a base. Another example is NaHSO4. In water the Na is again, dissociated with the ion and H2O comes in. They form HSO4 and an extra hydrogen (acid). This hydrogen finds some water and makes H3O.[1]
Hydrolysis of Metal and Nonmetal Oxides
[edit | edit source]These are a lot like Amphoteric ions except there simple metal and nonmetals instead of polyatomic ions. There is just a simple rule for these: If a metal ion is mixed with water, it makes a basic solution. If the compound is nonmetal, it forms an acid. [1]
Hydrolysis of Metal Ions
[edit | edit source]When a metal ion is mixed into a water solution, bonds with the water to form complex ions with the water. These will drop hydrogen in the water to make an acid.[1]
Predicting the Results of Hydrogen
[edit | edit source]There is a way to find out the concentration of an acid (usually hydrogen). See, in a reaction not all the chemicals react. If you mixed Na and Cl, you'd get a lot of NaCl and also some lone Na and Cl. This equation shows us what the concentration is: Ka× Kb=Kw. Say you have a reaction like HF + H2O → H3O + F. K is HF × H2O/H3O × F. Since H3O is an acid, K is replaced by Ka which is basically the same thing, it just specifies that we're finding the acid concentration. If we use Kb we are looking for a base. Kw is water. So say we have the reaction, CO3(aq) + H2O(aq) → OH(aq) + HCO3(aq). If we know that there is a 0.100 mol/L concentration of CO3 and the pH is 11.66. Since we are working with bases, we will use Kb. To find the concentration, we multiply each side of the equation and divide the sides. We divide the side of the reaction with the base by the side without the base. It looks like this:
- Kb = [OH] × [HCO3]/ [CO3]
Notice, we don't put water in the equation. This is because everything is dissolved in water and the CO3 concentration is measured in moles per liter of water. Now, let's put some numbers in the equation! We know the pH is 11.66, this means the pOH is 14 - 11.66 or 2.34 or 10-2.34. If we turn 10-2.34 into a proper exponent, we will know the concentration of OH. If you put 10-2.34 in your calculator, it will come out as the proper exponent: 4.57×10-3. This is the concentration of OH and since there is an equal concentration between OH and HCO3, the number is also the concentration of HCO3. Now we know the three numbers needed!
- Kb = (4.57 × 10-3)(4.57 × 10-3)/ 0.100
- Kb = 2.0 × 10-4
Measuring Acids and Bases
[edit | edit source]The pH Scale
[edit | edit source]Acids and bases are measured on something called the pH scale. It is a scale going from 0 to 14, where 1 is the most acidic and 14 is the most basic. 7 is in the middle of the scale, and so it is neutral.

Hydrogen Ion Concentration
[edit | edit source]The pH scale measures the relative concentration of hydrogen in a compound. Starting at seven, each level of the scale going up or down represents 10 times more concentration of hydrogen. Therefore, a pH level of 6 is ten times more acidic than one of 7, and a pH level of 5 is one hundred times more acidic than seven. Alternately, a pH level of 8 is ten times more basic than one of seven, and so on. This is what is known as a logarithm. In this case, it is a negative logarithm, because acids have a higher concentration of hydrogen than bases. This principle can be applied to help you know the pH from a hydrogen ion concentration. If you are given an Hydrogen concentration of 0.000006 M. You can find it's pH by converting it to scientific notation. The decimal place has to move back six times, so the scientific notation would be 6.0 x 10^-6. This means you multiply 6.0 by negative 10 six times, and therefore if it were a pH it would be one of 6. Reference: The virtual chembook (to be added as actual reference)
Naming Acids
[edit | edit source]Depending on what cation the hydrogen is attached to, acids will have different names. Simple acids, known as binary acids, have only one cation and one hydrogen. These acids are named by taking the prefix hydro, then putting the first syllable of the cation, then the suffix -ic. For example, HCl, which is hydrogen and chlorine, is hydrochloric acid. More complex acids are acids that have oxygen in the compound. There is a simple rule for these acids. Take nitrate (NO3) for an example. Any polyatomic ion having the suffix -ate uses the suffix -ic as an acid. So HNO3 will be nitric acid. When you have a polyatomic ion with one more oxygen than the -ate ion, then your acid will have the prefix per and the suffix -ic. With one less oxygen than the -ate ion, it will have the suffix -ous. With two less than the -ate ion, the prefix will be hypo and the suffix will be -ous. [2] Example: (some of these acids do not exist and are only used for example purposes)
Nitrate > HNO3 >Nitric Acid. One more Oxygen > HN2O4 >pernitric Acid. One less oxygen > HNO2 >Nitrous Acid. Two less oxygen > HNO > Hyponitrous Acid.
The Acid-base Concepts of Bronsted and Lowry
[edit | edit source]Both Bronsted and Lowry came up with a theory to explain how acids and bases work. Acids are compounds that will drop hydrogen ions. These loose hydrogens will proceed to bond onto other compounds. Bases are the opposite; they pick up hydrogen ions. Stronger acids drop hydrogens easier than weak acids. Strong bases take hydrogen ions more than weak bases. The really strong bases will take hydrogens from compounds that don't want to give up their hydrogens.[1]
Conjugate Acids and Bases
[edit | edit source]After a strong acid gives up its hydrogen, it becomes a weak base. Redundantly, a strong base becomes a weak acid after it gets a hydrogen. There is a simple explanation for this. An acid has a hydrogen and has a bond with it. The bind may be weak, but it is a bond none the less. After the acid get rid of the hydrogen, it doesn't really want to get another one, but it does have room for the bond. It works just opposite to that with bases.[1]
Acid-base Indicators
[edit | edit source]When a substance changes colour when it is mixed with an acid or base, it is called an acid-base indicator. Litmus paper is the most common type. When you put a piece of litmus paper in an acid, it turns red. When you put it in a base, it turns blue. Remember: Acid Red, Base Blue.[1]
Buffers
[edit | edit source]A buffer is a solution that will keep something at about the same pH. For an acid, it is a weak base and for a base, it is a weak acid. When you mix the buffer to an acid so the solution is about half acid and half buffer, it is acting as a buffer. During this time, the acid is giving its hydrogen ions to the base buffer. However, when all the hydrogen ions are taken by the buffer, the solution reaches the buffer capacity. Then, if you add any more of the buffer, the pH will just go up to the pH of the buffer.[1]
References
[edit | edit source]
Quantitative Relationships in Chemical Changes
In quantitative relationships, you can use a balanced chemical equation and knowledge about how much of a substance there is to find out what substance you will have too much of, what you will run out of and how much of a substance you will need. Limiting Reagent is the substance that runs out, and excess reagent is the substance you have leftovers of.
Gravimetric Stoichimetry
[edit | edit source]Gavemeter is determining solid reactants by weight.
Gas Stoichimetry
[edit | edit source]When using gas stoichmetry determine the amount of gas used by volume. The STP (standard temperature and pressure) is 22.4 L/mol. The SATP (standard ambient temperature and pressure) is 24.8 L/mol. A formula you can use with gas stochimetry is pv=nRT. 'p' is pressure, 'v' is volume, n is moles (not moler mass) 'R' is the constant (8.31 kPa×L/mol × Kalvin) and 'T' is the temperature in Kalvin. You use this formula to find any one of the reactants.
Chemical Analysis By Titration(Solution Stoichimetry)
[edit | edit source]Titration is determining liquid reactants by mol/L. In titration, we know the concentration of one the reactants. One way of finding the concentration of one of the reactants is by adding the reactant with the known concentration to the reactant with the unknown concentration a little bit at a time using a buret. When the reactant with the unknown concentration fully reacts with the other reactant, you can check the buret to see how much known reactant you used. then look at the balanced chemical equation to calculate the concentration of the reactant.
