General Chemistry/Chemistries of Various Elements/Transition Metals
Transition Metals
[edit | edit source]The transition metals are found in the middle of the periodic table. There are two definitions of transition metals:
- All d-block elements (Groups 3-12)
- Elements with partially occupied d-orbitals or that can form cations with partially occupied d-orbitals
The first definition is more common and is used casually, but the second definition emphasizes the unique properties of transition metals and is the one used by IUPAC (The International Union of Pure and Applied Chemistry). The second definition is commonly considered to exclude Zn, Cd and Hg because these elements have a d10 electronic configuration (the d-orbitals being fully, not partially, occupied). However, recently reported fluoride Hg(IV) compounds, which have a d8 configuration put this exemption into doubt and make it reasonable to consider Hg (and possibly Zn and Cd too) as transition metals.
Transition metals behave differently than other metals because of their partially occupied d-orbitals. Adding electrons to a transition metal does not affect its valence shell because the electrons go into the d-orbital (which is not part of the valence shell). All transition metals have one or two valence electrons.
Electrons and Oxidation
[edit | edit source]Transition metals are interesting because they can have several oxidation states, unlike most other metals. This happens because the transition metals can lose their d electrons in addition to their s electrons when forming ions.

Remember that an electron orbital is most stable when it is full or half-full (or empty). Studying the electron configurations of the transition metals shows an interesting pattern:
| Sc: | [Ar]4s23d1 |
| Ti: | [Ar]4s23d2 |
| V : | [Ar]4s23d3 |
| Cr: | [Ar]4s13d5 |
| Mn: | [Ar]4s23d5 |
| Fe: | [Ar]4s23d6 |
| Co: | [Ar]4s23d7 |
| Ni: | [Ar]4s23d8 |
| Cu: | [Ar]4s13d10 |
| Zn: | [Ar]4s23d10 |
To be more stable, an s-electron "jumps up" to the d-orbital in chromium and copper. This also occurs in in heavier transition metals like molybdenum, tungsten, and platinum. With heavier transition metals in Periods 5, 6, and 7, the effects of relativity cause changes in the energy levels of the orbitals. In those elements, s-electrons "jump up" to d-shells more often than expected with the full/half-full rule.
When ions of the transition metals form, they lose their s-electrons first, then they lose their d-electrons if further ionized. For example, copper can form two different ions, and titanium can form three:
| Cu: | [Ar]4s13d10 |
| Cu+: | [Ar]4s03d10 |
| Cu2+: | [Ar]4s03d9 |
| Ti: | [Ar]4s23d2 |
| Ti2+: | [Ar]4s03d2 |
| Ti3+: | [Ar]4s03d1 |
| Ti4+: | [Ar]4s03d0 |
Colors
[edit | edit source]Transition metals and their oxides, when dissolved, form colored compounds. Group 1 and 2 metals are clear when dissolved and white when precipitated. Other metals, like lead, are clear when dissolved and may have color when precipitated (lead precipitates are yellow). Transition metals, on the other hand, are colored when dissolved. Different metals are known for their specific colors, finding use as inks or paints.

Families
[edit | edit source]Coinage Metals
[edit | edit source]The "coinage metals" are copper, silver, gold, and roentgenium. These elements are used for much more than just coins, and many other elements besides these are made into coins. Furthermore, roentgenium is radioactive with a half-life of 3.6 seconds, making it useless for commercial applications. Consequently, the "coinage metals" are more appropriately called Group 11 (IB) elements.
Copper, silver, and gold, although relatively rare (copper), rare (silver), or extremely rare (gold), are among the longest-known and most familiar elements. They are soft, shiny, dense metals resistant to corrosion and very good conductors of electricity. Roentgenium, a recently discovered synthetic element, is so short-lived that its physical and chemical properties are ill-defined.
Copper is by far the most heavily used of these elements due to its electrical properties, its commonness (contrasted to silver and gold) and the attractiveness of its alloys brass and bronze. Until aluminum became commonplace, copper was second only to iron in production among the metals.
-
Copper pipes
-
Silver, the shiniest
-
Gold
They are easy to identify when found because copper (reddish) and gold (yellow) are the only two colored metals that people are likely to encounter. Silver is the shiniest of metals, and it is usually found in the presence of copper or gold and gives an obvious contrast. They are often found uncombined.
Because of their softness they are easily struck as coins, and their comparative rarity and attractiveness, along with their resistance to corrosion make them compact stores of wealth. They are too soft to have structural value, but copper alloys with such elements as zinc and tin to form harder brasses and bronzes. Brass and bronze were essential in the earliest metal tools; without them, civilization as we know would be impossible. Gold and silver, due to their attractiveness and their resistance to oxidation, have been used heavily in jewelry and other ornamental works. Gold, although extremely expensive, is so malleable that at modest cost a small amount can be pounded into a foil of extreme thinness that allows it to be used as a covering of some architectural objects; a little gold goes a long way.
Copper oxidizes with some difficulty to the +1 state in halides and an oxide and to the +2 state in salts such as copper sulfate CuSO4. Soluble copper compounds are easily identified by their distinctive blue-green color. Silver oxidizes to the +1 state in such substances as silver nitrate AgNO3 and silver sulfide Ag2S, the latter the typical blackening of silver. Gold oxidizes to the +1 and +3 state with great difficulty.
These elements are poor (copper) to extremely-poor (gold) reducing agents and their compounds are very good oxidizing agents. Copper ions oxidize most metals:
The reaction is even stronger with either silver or gold. In effect a solution of one of these metals' salts plates most other metals.
Silver is most electrically conductive metal, followed by copper then gold. This makes copper a favorite material for electrical wires. Gold-tipped wires are employed in situations that need electrical precision (like high-quality audio) because gold will not tarnish (the tarnish of copper is much less conductive).
Zinc Family
[edit | edit source]The Zinc Family is Group 12 (IIB) and consists of zinc, cadmium, mercury, and copernicum.
Zinc, cadmium, and mercury are metals with low melting points for metals. This is because they have an especially stable electron configuration. Mercury is so poor at forming metallic bonds that it is liquid at room temperature.
-
A zinc coin
-
Cadmium pieces
-
A puddle of mercury
Zinc and cadmium are soft metals that easily oxidize to the +2 oxidation state. Neither of these two metals appears uncombined in nature. Zinc is heavily used in alloys with copper to create a harder metal known as brass; as a coating for iron (the process is called "galvanizing"), it oxidizes to form a protective layer of zinc oxide (ZnO) that protects the iron from oxidation, also known as rust. Zinc oxide is much safer than lead oxide and is often used in white paint. Since 1982, zinc has been the main metal used in American pennies. It is now used in new organ pipes.
Cadmium forms two substances, cadmium yellow (cadmium sulfide, CdS) and cadmium red (cadmium selenide, CdSe) that appeared in paints. These paints had strong colors that many of the great artists of the Impressionist periods cherished in their paintings. But these substances are very poisonous, and painters who used them often died young and crippled. Modern painters ordinarily use different paints that do not use these two poisonous chemicals.
Mercury, in contrast, is a shiny liquid at room temperature and oxidizes with some difficulty. It conducts electricity well. Because it is liquid it is an unusual metal—but it is a metal. It has been used in thermometers (but not so often after it has been identified as a dangerous poison) because it expands with heat and in switches where it can flow into a closed space to close a circuit. Mercury oxidizes to the +2 state in mercuric chloride (HgCl2); in some strange compounds, two mercury atoms share an electron and offer their "spare" electrons to form substances in the +1 state, such as mercurous chloride (Hg2Cl2).
Zinc is an essential trace element for living things; it has some germicidal properties and is toxic (poisonous) in large quantities. Zinc pennies should never be swallowed. Cadmium, mercury, and their compounds are very dangerous poisons. Although mercury is attractive and has remarkable properties, it should be used with extreme care, and only by workers who have appropriate knowledge of its hazards.
The artificial Element 112 named copernicum in 2010 is probably part of this group in its properties, but it is extremely difficult to produce and too unstable to have a well-defined chemistry. Few atoms of this element have ever been made.
The elements of Groups 8, 9, and 10 are in two distinct groups: the common elements iron, cobalt, and nickel of the upper row of transition metals and the platinum metals of the second and third rows, and the far-scarcer platinum metals of the two lower rows of transition elements.
Iron, cobalt, and nickel
[edit | edit source]These elements are fairly-good reducing agents -- so good that they rarely appear uncombined in nature. Iron is by far the most common of these. One of the most common elements in the universe, it is the heaviest metal that forms in normal fusion in stars (but only the largest stars). Once a star begins to produce iron in its core, that star is doomed in short order to a violent explosion that destroys the star and scatters its matter, including all of the elements that it has formed in fusion.
Uncombined iron, cobalt, and nickel -- but especially iron -- are to be found in meteors, solid objects that strike the earth. Iron is by far the most common of the transition elements, and one of the most useful. It's hard to count all the uses of iron, the metal most used (whether pure or in alloys) in almost all machines. Giant "glass box" skyscrapers depend upon iron bars within their concrete "skeletons" to give them strength and stability. The rails of railroads are long iron bars. Concrete highways and airstrips have iron re-enforcing bars to give them the strength to hold heavy vehicles. The vehicles themselves are largely iron and a harder material known as steel, an alloy of iron, carbon, and often metals other than iron.
Iron is the cheapest of all structural metals. With some skill of an artisan known as a blacksmith it can be worked into many useful objects such as horseshoes, nails, plows, chains, pails, ladders, and many tools. In foundries, iron and steel are shaped in far greater quantities into such objects as furniture and parts of aircraft, ships, motor vehicles, and appliances.
Iron has one fault as a structural material: it rusts easily. In the presence of water (especially salt water) it corrodes into oxides:
Fe(s) + 1/2 O2(g) → FeO(s) 2 Fe(s) + 3/2 O2(g) → Fe2O3(s)
and a mixed oxide known as hematite
2 Fe(s) + 3/2 O2(g) → Fe2O3(s)
one of the most common ores of iron. Iron oxides are mildly alkaline, so iron resists attacks by alkalis; acids attack it. For example,
Fe (s) + H2SO4(l) → Fe2+(aq) + SO42-(aq) + H2(g)
Even a comparatively weak acid, like phosphoric acid, can attack iron oxide. This is the "naval jelly" reaction that removes rust from iron:
FeO(s) + H3PO4(l) → Fe2+(aq) + HPO4(aq)-2 + H2O(l)
A great advance of humanity, the beginning of the Iron Age, began when people found that they could separate iron from oxygen by burning it with carbon (usually charcoal) which can reduce iron oxides to iron:
Fe3O4(s) + 4 C(s) → 3 Fe(s) + 4 CO(g)
Much of existing economic activity depends upon the extraction of iron ore, the reduction of iron ore to iron, the strengthening of iron to steel, the creation of iron and steel objects, and the various practices used in protecting iron from corrosion.
Important as that activity is, our lives would be impossible without an important compound of iron known as hemoglobin which carries oxygen through the bloodstream to cells where the cells can use the oxygen to release energy from food also delivered to cells through the bloodstream.
-
two suspension bridges
-
an old iron plow
-
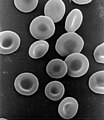
red blood cells contain hemoglobin
-
an electromagnet attracting scrap iron
The earth itself has a hot, dense core of largely iron and nickel. At the temperatures characteristic of the Earth's core the iron and nickel form a giant natural magnet that creates a magnetic field that goes beyond the Earth itself into the atmosphere. That magnetic field drives off much dangerous radiation that would kill life on the Earth's surface if it reached the Earth's surface.
Cobalt and nickel are both far scarcer than iron and not as extensively used in commerce as iron, although they have specialized uses.
Platinum Family
[edit | edit source]The Platinum group metals are ruthenium, rhodium, palladium, osmium, iridium, and platinum. These elements are found in the second two rows of Groups 8/9/10 (IIIB).
Unlike their lighter counterparts in Groups 8, 9, and 10 of these elements are resistant to corrosion and tarnish. They serve as catalysts for many chemical reactions, speeding up the reaction without being consumed by it.
Palladium, osmium, and the other platinum group metals absorb hydrogen when powdered.
Rhodium is used in catalytic converters—metallic structures found inside vehicles. Catalytic converters convert nitric oxides (which are toxic pollutants) into elemental nitrogen and oxygen (both of which make up breathable air):
That reaction would not occur without rhodium to serve as a catalyst.