High School Chemistry/Electron Configurations
In the last section, we determined that the members of the chemical families (vertical columns on the periodic table) all have the same electron configuration in their outermost energy levels. In the next section, we will review the orbital representation for electron configuration and the electron configuration code. Then we will learn a shortcut for writing electron configuration codes.
Lesson Objectives
[edit | edit source]- Convert from orbital representation diagrams to electron configuration codes.
- Distinguish between outer energy level (valence) electrons and core electrons.
- Use the shortcut method for writing electron configuration codes for atoms and ions.
Shortcut for Writing Electron Configurations
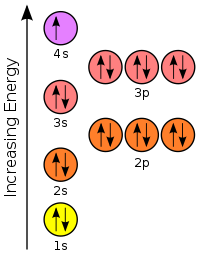
[edit | edit source]The diagram below represents the orbital representation diagram used in earlier chapters. The orbital representation diagram has a few different variations but all are used to draw electron configurations. Most show the orbitals in groups, as lines, boxes, or circles with each orbital having its own line (or circle) within each sublevel.
The sublevel 2p has 3 orbitals (2px, 2py, and 2pz) and each of these orbitals has its own line (or box).
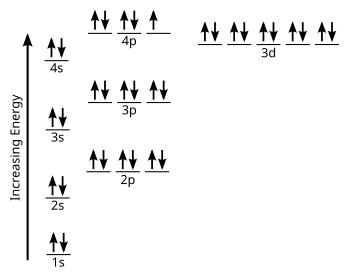
Let's begin this section with the orbital box (or the orbital representation diagram) for a neutral atom. Draw the electronic configuration for potassium using the electron configuration diagram below. Remember that potassium is element number 19 so has 19 electrons. Every line in the energy diagram below holds 2 electrons of opposite spins.
Now we can simplify this by excluding all of the levels and sublevels that are not in use or do not hold electrons.
Writing the electron configuration code for potassium, we would get:
- :
Remember, the noble gases are the elements that are nearly non-reactive, partially due to the fact that the outermost energy level electron configuration was full (ns2np6). Look at potassium on the Periodic Table and then find the preceding noble gas. Drag your finger counting backward from potassium to the nearest noble gas. What do you get? Argon (Ar) is correct. Now, let's draw the electron configuration for argon and place the electron configuration for potassium beside it.
What do you notice looking at the two diagrams? Potassium has the same electron configuration as an argon +1 electron. We can make this point when we write electronic configuration codes.
: :
Therefore,
- : (This is known as the noble gas configuration code system or the "short-cut")
Let's try another. Draw and write the electron configuration of gallium (Ga). Gallium is number 31 and therefore has 31 electrons. It is also in the 4th period, the third element in the period of main group elements. From this information, we know that the outermost electrons will be 4s24p1.
Writing the electron configuration code for gallium, we get 1s22s22p63s23p64s23d104p1. In numerical order, the electronic configuration for gallium is 1s22s22p63s23p63d104s24p1.
Now, go back to the previous noble gas. Again, we find the previous noble gas is argon. Remember argon has electronic configuration of 1s22s22p63s23p6.
: :
Therefore,
- :
This seems to work well for neutral atoms, what about ions? Remember that a cation is formed when electrons are removed from a neutral atom and an anion is formed when electrons are added to a neutral atom. But where do the electrons get added to or removed from? The electrons that are involved in chemical reactions are the ones that are in the outer shell. Therefore, if electrons are to be removed or added, they get removed from or added to the shell with the highest value of n.
Let's now look at an example. Draw the electron configuration for Fe. Then, write the reaction for the formation of Fe2+.
The reaction for the formation of the Fe2+ ion is: Fe → Fe2+ + 2e−. Therefore we have to remove 2 electrons. From where do we remove the two electrons? The rule is to remove from the highest n value first which, in this case, means we remove from the electrons from the 4s sublevel first.
Remember that when we write electron configuration codes using noble gas configurations we use the previous noble gas. In writing the electron configuration code for Fe or Fe2+, we would use the symbol [Ar] to substitute for 1s22s22p63s23p6.
In addition to forming Fe2+ ions, iron atoms are also capable of forming Fe3+ ions. Extra stability is gained by electron configurations that have exactly half-filled d orbitals. The third electron that is removed from an iron atom to form the Fe3+ ion is removed from the d orbital giving this iron ion a d orbital that is exactly half-filled. The extra stability of this configuration explains why iron can form two different ions.
Now what about negative ions, the anions? Remember that negative ions are formed by the addition of electrons to the electron cloud. Look at bromine, Br. Neutral bromine has atomic number 35 and therefore, holds 35 electrons.
The reaction for the formation of the Br− ion is: Br + e− → Br−. The bromide ion is formed by the addition of one electron. If you look at the electron configuration for bromine, you will notice that the 4p sublevel needs one more electron to fill the sublevel and if this sublevel is filled, the electron configuration for Br− is the same as that of the noble gas krypton (Kr). When adding electrons to form negative ions, the electrons are added to the lowest unfilled n value.
or
Notice how Br− has the same electron configuration as Kr. When this happens, the two are said to be isoelectronic.
|
Sample Question Write the electron configuration codes for the following atoms or ions using the noble gas electron configuration shortcut.
Solution:
|
Core Electrons
[edit | edit source]When we look at the electron configuration for any element we can distinguish that there are two different types of electrons. There are electrons that work to do the reacting and there are those that don't. The outer shell electrons are the valence electrons and these are the electrons that are responsible for the reactions that the atoms undergo. The number of valence electrons increases as you move across the periodic table for the main group elements.
In older periodic tables, the groups were numbered using an A and B group numbering system. The representative groups (s and p blocks) were numbered with A and the transition elements were numbered with B. The table below has both numbering systems. The A numbering is shown for you in the diagram in red; this is all we will be concerned with for this lesson. The number of valence electrons in the A groups increases as the group number increases. In fact, the number of the A groups is the number of valence electrons. Therefore, if an element is in group 3A, it has 3 valence electrons, if it is in group 5A, it has 5 valence electrons.
All electrons other than valence electrons are called core electrons. These electrons are in inner energy levels. It would take a very large amount of energy to pull an electron away from these full shells, hence, they do not normally take part in any reactions. Look at the electron configuration for gallium (Ga). It has 3 valence electrons and 28 core electrons.
|
Sample Question How many core electrons and valence electrons are in each of the following?
Solution:
|
Lesson Summary
[edit | edit source]- An orbital box diagram can be used to draw electron configurations for elements.
- The noble gas configuration system is the shorthand method for representing the electron configuration of an element.
- The noble gas symbol represents all of, or most of, the core electrons.
- For cations, the electrons are removed from the outermost sublevel having the greatest n value.
- For anions, the electrons are added to the unfilled energy level having the lowest n value.
- Isoelectronic species have the same electron configurations.
- Using an orbital box diagram, the core electrons and the valence electrons can easily be visualized.
- The valence electrons are those electrons that are in the outermost shell.
Review Questions
[edit | edit source]- What is the difference between the standard electron configuration code and the electron configuration code using noble gas configurations?
- The standard electron configuration is often called the ground state electron configuration. Why do you think this is so?
- How is it possible to have the same number of core electrons and valence electrons for two different ions?
- Draw the orbital representation for the electron configuration of potassium, K.
- Write the electron configuration code for potassium, K.
- Write the noble gas electron configuration code for potassium, K.
- How many core electrons and valence electrons are in potassium, K?
- Write the electron configuration for potassium, K+.
- Write the noble gas electron configuration code for potassium, K+.
- With what species is K+ isoelectronic?
- The electron configuration below is for which element?
- [Kr]4d105s25p2
- (a) Sb
- (b) Sn
- (c) Te
- (d) Pb
- The electron configuration below is for which element?
- [Xe]5d106s26p4
- (a) Pb
- (b) Bi
- (c) Po
- (d) Tl
- What is the noble gas electron configuration for the bromine element?
- (a) 1s22s22p63s23p64s24p5
- (b) 1s22s22p63s23p63d104s24p5
- (c) [Ar]4s24p5
- (d) [Ar]3d104s24p5
- Write the noble gas electron configuration for each of the following.
- (a) Al
- (b) N3−
- (c) Sr2+
- (d) Sn2+
- (e) I
- How many core electrons and valence electrons are in each of the following?
- (a) Mg
- (b) C
- (c) S
- (d) Kr
- (e) Fe
Vocabulary
[edit | edit source]- core electrons
- Electrons that occupy energy levels below the outermost energy level.
- isoelectronic
- Having the same electron configuration.
- orbital box diagram
- A diagram for drawing the electron configurations where sub-levels are shown in groups (or even in boxes) and each orbital has its own line (or box) within each sub-level.
- valence electrons
- Electrons that occupy the outer shell of the atom or ion.
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License










![{\displaystyle [{\text{Ar}}]4s^{1}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b1c4b17922ea1878953e29b582e36ad2da592cc7)



![{\displaystyle [{\text{Ar}}]4s^{2}3d^{10}4p^{1}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1d1ab7511907f596545a2b6121a960dabb4f6895)






![{\displaystyle [{\text{Ar}}]3d^{6}4s^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5d9afad4dcec6812aff77aebaf74882154c0c274)
![{\displaystyle [{\text{Ar}}]3d^{6}+2e^{-}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/158efdfbfd97872dbaadd3d7af410da550d4751c)

![{\displaystyle [{\text{Ar}}]3d^{5}+3e^{-}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d42dc0fab3d7bf87bf2e055cd223842ae7b729cd)




![{\displaystyle [{\text{Kr}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/efe1422248466f512bd18f7076dfda35b64ee1f3)
