High School Chemistry/Ionization Energy
When we study the trends in the periodic table, we cannot stop at just atomic size. In this section of the chapter, we will begin an understanding of an important concept, namely ionization energy and recognize its trend on the Periodic Table.
Lesson Objectives
[edit | edit source]- Define ionization energy.
- Describe the trend that exists in the Periodic Table for ionization energy.
- Describe the ionic size trend that exists when elements lose one electron.
Ionization Energy is the Energy Required to Remove an Electron
[edit | edit source]Lithium has an electron configuration of 1s22s1. Lithium has one electron in its outermost energy level. In order to remove this electron, energy must be added to the system. Look at the equation below:
With the addition of energy, a lithium ion can be formed from the lithium atom by losing one electron. This energy is known as the ionization energy. The ionization energy is the energy required to remove the most loosely held electron from a gaseous atom or ion. "In the gaseous phase" is specified because in liquid or solid, other energies get involved. The general equation for the ionization energy is as follows.
The higher the value of the ionization energy, the harder it is to remove that electron. We can see a trend when we look at the ionization energies for the elements in period 2. Table 10.7 summarizes the electron configuration and the ionization energies for the elements in the second period.
| Element | Electron Configuration | First Ionization Energy IE1 |
|---|---|---|
| Lithium (Li) | [He]2s1 | 520 kJ/mol |
| Beryllium (Be) | [He]2s2 | 899 kJ/mol |
| Boron (B) | [He]2s22p1 | 801 kJ/mol |
| Carbon (C) | [He]2s22p2 | 1086 kJ/mol |
| Nitrogen (N) | [He]2s22p3 | 1400 kJ/mol |
| Oxygen (O) | [He]2s22p4 | 1314 kJ/mol |
| Fluorine (F) | [He]2s22p5 | 1680 kJ/mol |
When we look closely at the data presented in Table 10.7, we can see that as we move across the period from left to right, in general, the ionization energy increases. At the beginning of the period, with the alkali metals and the alkaline earth metals, losing one or two electrons allows these atoms to become ions.
As we move across the period, the atoms become smaller which causes the nucleus to have greater attraction for the valence electrons. Therefore, the electrons are more difficult to remove.
A similar trend can be seen for the elements within a family. Table 10.8 shows the electron configuration and the first ionization energies (IE1) for some of the elements in the first group, the alkali metals.
| Element | Electron Configuration | First Ionization Energy IE1 |
|---|---|---|
| Lithium (Li) | [He]2s1 | 520 kJ/mol |
| Sodium (Na) | [Ne]3s1 | 495.5 kJ/mol |
| Potassium (K) | [Ar]4s1 | 418.7 kJ/mol |
Comparing the electron configurations of lithium to potassium, we know that the electron to be removed is further away from the nucleus. We know this because the n value is larger meaning the energy level where the valence electron is held is larger. Therefore it is easier to remove the most loosely held electron because the atom is larger with a greater shielding effect which means that the nucleus has less control over potassium's outer electron, 4s1.
Therefore IE1 for potassium (418.7 kJ/mol) is less than IE1 for lithium (520 kJ/mol).
If a second electron is to be removed from an atom, the general equations are the following:
Since there is an imbalance of positive and negative charges when a second electron is being removed, the energy required for the second ionization (IE2) will be greater than the energy required for the first ionization (IE1). Simply put, IE1 < IE2 < IE3 < IE4.
The Charge on the Nucleus Increases and Size Decreases
[edit | edit source]So if we look at the ionization energy trend in the Periodic Table and add it to the trend that exists with atomic size we can show the following on our Periodic Table chart.

But why does the ionization energy increase going across a period and decrease going down a group? It has to do with two factors. One factor is that the atomic size decreases. The second factor is that the effective nuclear charge increases. The effective nuclear charge is the charge experienced by a specific electron within an atom. Remember, the nuclear charge was used to describe why the atomic size decreased going across a period. When we look at the data in Table 10.7 again, we can see how the effective nuclear charge increases going across a period. Table 10.9 shows the effective nuclear charge along with the ionization energy for the elements in Period 2.
| Element | Electron Configuration |
# of protons |
# of core electrons |
Effective nuclear charge |
Ionization Energy |
|---|---|---|---|---|---|
| Lithium (Li) | [He]2s1 | 3 | 2 | 1 | 520 kJ/mol |
| Beryllium (Be) | [He]2s2 | 4 | 2 | 2 | 899 kJ/mol |
| Boron (B) | [He]2s22p1 | 5 | 2 | 3 | 801 kJ/mol |
| Carbon (C) | [He]2s22p2 | 6 | 2 | 4 | 1086 kJ/mol |
| Nitrogen (N) | [He]2s22p3 | 7 | 2 | 5 | 1400 kJ/mol |
| Oxygen (O) | [He]2s22p4 | 8 | 2 | 6 | 1314 kJ/mol |
| Fluorine (F) | [He]2s22p5 | 9 | 2 | 7 | 1680 kJ/mol |
The electrons that are shielding the nuclear charge are the core electrons, or in the case of Period 2, the 1s2 electrons. The effective nuclear charge is the difference between the total charge in the nucleus (the number of protons) and the number of shielded electrons. Notice how as the effective nuclear charge increases, so does the ionization energy. Overall the general trend for ionization energy is shown below.
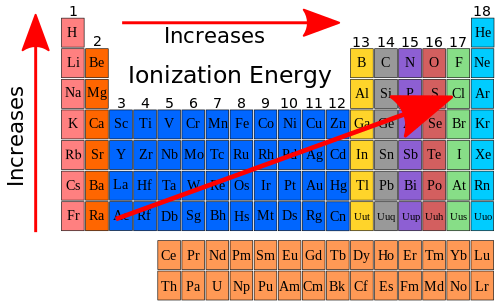
|
Sample Question What would be the effective nuclear charge for Cl? Would you predict the ionization energy to be higher or lower than fluorine? Solution: Chlorine has the electronic configuration of [Ne]3s23p5 The effective nuclear charge is 7, the same as fluorine. Predicting the ionization energy would be difficult with this alone. The atomic size, however, is larger for chlorine than for fluorine because now there are three energy levels (chlorine is in Period 3). Now we can say that the ionization energy should be lower than that of fluorine because the electron would be easier to pull off the level further away from the nucleus. (Indeed, the value for chlorine is 1251 kJ/mol). |
Some Anomalies With the Trend in Ionization Energy
[edit | edit source]There are a few anomalies that exist with respect to the ionization energy trends. Going across a period there are two ways in which the ionization energy may be affected by the electron configuration. When we look at period 3, we can see that there is an anomaly observed as we move from the 3s sublevels to the 3p sublevel. The table below shows the electron configurations for the main group elements in period 3 along with the first ionization energy for these elements.
|
In the table we see that when we compare magnesium to aluminum the first IE decreases instead of increasing as we would have expected. So why would this be so? Magnesium has its outermost electrons in the s sublevel. The aluminum atom has its outermost electron in the 3p sublevel. Since p electrons have just slightly more energy than s electrons, it takes a little less energy to remove that electron from aluminum. One other slight factor is that the electrons in 3s2 shield the electron in 3p1. These two factors allow the IE1 for Al to be less than IE1 for Mg.
When we look at the table below, we can see that the ionization energy for nitrogen seems out of place.
|
While nitrogen has one electron occupying each of the three p orbitals in the 2nd sublevel, oxygen has two orbitals occupied by only one electron but one orbital containing a pair of electrons. The greater electron-electron repulsion experienced by these 2p electrons allows for less energy to be needed to remove one of these. Therefore, IE1 for oxygen is less than nitrogen.
Lesson Summary
[edit | edit source]- Ionization energy is the energy required to remove the most loosely held electron from a gaseous atom or ion. Ionization energy generally increases across a period and decreases down a group. The effective nuclear charge is the charge of the nucleus felt by the valence electron.
- The effective nuclear charge and the atomic size help explain the trend of ionization energy. Going down a group the atomic size gets larger and the electrons can be more readily removed, therefore, ionization energy decreases. Going across a period the effective nuclear charge increase so the electrons are harder to remove and the ionization energy increases. Once one electron has been removed, a second electron can be removed but IE1 < IE2. If a third electron is removed, IE1 < IE2 < IE3 and so on.
Review Questions
[edit | edit source]- Define ionization energy and show an example ionization equation.
- Draw a visual representation of the periodic table describing the trend of ionization energy.
- Which of the following would have the largest ionization energy?
- (a) Na
- (b) Al
- (c) H
- (d) He
- Which of the following would have the smallest ionization energy?
- (a) K
- (b) P
- (c) S
- (d) Ca
- Place the following elements in order of increasing ionization energy: Na, O, Ca, Ne, K.
- Place the following elements in order of decreasing ionization energy: N, Si, S, Mg, He.
- Using experimental data, the first ionization energy for an element was found to be 600 kJ/mol. The second ionization energy for the ion formed was found to be 1,800 kJ/mol. The third ionization energy for the ion formed was found to be 2,700 kJ/mol. The fourth ionization energy for the ion formed was found to be 11,600 kJ/mol. And finally the fifth ionization energy was found to be 15,000 kJ/mol. Write the reactions for the data represented in this question. Which group does this element belong? Explain.
- Using electron configurations and your understanding of ionization energy, which would you predict would have higher second ionization energy: Na or Mg?
- Comparing the first ionization energy (IE1) of calcium, Ca, and magnesium, Mg, :
- (a) Ca has a higher IE1 because its radius is smaller.
- (b) Mg has a higher IE1 because its radius is smaller.
- (c) Ca has a higher IE1 because its outer sub-shell is full.
- (d) Mg has a higher IE1 because its outer sub-shell is full.
- (e) they have the same IE1 because they have the same number of valence electrons.
- Comparing the first ionization energy (IE1) of beryllium, Be, and boron, B:
- (a) Be has a higher IE1 because its radius is smaller.
- (b) B has a higher IE1 because its radius is smaller.
- (c) Be has a higher IE1 because its s sub-shell is full.
- (d) B has a higher IE1 because its s sub-shell is full.
- (e) They have the same IE1 because B has only one more electron than Be.
Vocabulary
[edit | edit source]- effective nuclear charge
- The charge on the atom or ion felt by the outermost electrons (valence electrons).
- ionization energy
- The energy required to remove an electron from a gaseous atom or ion: energy + J → J+ + e− (first ionization energy).
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License









![{\displaystyle [{\text{He}}]+2s^{1}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6ef0c3bd08060b641f187157e1f50ebddedd4525)
![{\displaystyle [{\text{He}}]+e^{-}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/eea03da7708e973f2218af8fc0a9c7f23c226b03)
![{\displaystyle [{\text{Ne}}]+3s^{2}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/79706974a36cef696a78dc6c3ef8d64d4b2cd90b)
![{\displaystyle [{\text{Ne}}]+2e^{-}\,\!}](https://wikimedia.org/api/rest_v1/media/math/render/svg/25977b668258576b378bb0118a84e86ccf5fbb84)







