High School Chemistry/Schrodinger's Wave Functions
In the last lesson, you learned that electrons and, in fact, all objects with mass, have wavelike properties. It might be tempting to visualize matter waves as being just like ocean waves, or waves in a puddle, but it turns out that matter waves are special. Unlike ocean waves or puddle waves, matter waves are "trapped" in space and, as a result, can never die out, escape, or disappear. If you think carefully, you'll realize that this isn't true of most other waves with which you are familiar. You can form waves in a puddle by stirring the puddle with a stick. When you do, what you'll notice is that the waves you create actually move from your stick out to the edge of the puddle, where they disappear. As long as you disturb the puddle with your stick, the puddle will have waves in it. But as soon as you leave the puddle alone, the surface of the puddle will become as calm as glass. Matter waves aren't like that. Unlike puddle waves, which eventually die out as they escape from the puddle, matter waves never do, because matter waves don't move. As a result, they are forever trapped in the matter that holds them. We'll talk more about these special matter waves in the next section.
Lesson Objectives
[edit | edit source]- Distinguish between traveling and standing waves.
- Explain why electrons form standing waves, and what this means in terms of their energies.
- Define an electron wave function and electron density and relate these terms to the probability of finding an electron at any point in space.
An Electron is Described as a Standing Wave
[edit | edit source]Most of the waves that you're probably familiar with are known as traveling waves, because they travel or move. When you're sitting on your surfboard, trying to catch a good wave, you'll often look out to sea in the hopes of spotting a "big one" (Figure 6.3). When you finally do, you know that even though the big wave may be quite a distance off, it will eventually arrive at your surfboard and carry you in to shore. This, of course, implies that ocean waves are traveling waves because they actually move through the water. Similarly, if you're in the stands watching the Oakland As play ball, you might find yourself jumping up and cheering as "the wave" passes through the stadium (Figure 6.4). Again, this is an example of a traveling wave, because it moves from fans at one end of the stadium to fans at the other. There are, however, special waves that stay in one spot. Scientists call these waves standing waves.


In an earlier part of this text, a wave was described in which a rope was tied to a tree and a person jerked the other end of the rope up and down to create a wave in the rope. When a wave travels down a rope and encounters an immoveable boundary (like a tree), the wave reflects off the boundary and travels back up the rope. This causes interference to occur between the wave traveling toward the tree and the reflected wave traveling back toward the person. If the person adjusts the rhythm of their hand just right, they can arrange for the crests and troughs of the wave moving toward the tree to exactly coincide with the crests and troughs of the reflected wave. When this occurs, the apparent horizontal motion of the wave ceases and the wave appears to "stand" in the same place in the rope. This is called a standing wave. In such a case, the crests and troughs will remain in the same places and nodes will appear between the crests and troughs where the rope does not appear to move at all.
In the standing wave shown in Figure 6.5, the positions of the crests and troughs remain in the same positions. The crests and troughs appear to exchange places above and below the center line of the rope. The flat places where the rope crosses the center axis line are called nodes (positions of zero displacement). These nodal positions do not change. Traveling waves appear to travel, and standing waves appear to stand still.

Even though standing waves don't move themselves, they are actually composed of traveling waves that do. Standing waves form when two traveling waves traveling in opposite directions at the same speed combine or run into each other. In today's lab, you'll learn how to create standing waves in a jump rope by feeding traveling waves into the jump rope from opposite directions. Even though a standing wave doesn't move, it can still "die out". As soon as the traveling waves that form a standing wave disappear, so does the standing wave itself. You'll see this first hand in the jump rope experiment. When you stop flicking the jump rope, the jump rope slackens and the standing waves are gone.
Why, then, are standing waves often associated with "trapped" waves, or waves that never die out? The connection between standing waves and trapped waves isn't a misconception or a misunderstanding. It turns out that standing waves almost always form when traveling waves are "trapped" in a small region of space. Imagine what would happen if you took a whole train of traveling waves, locked them up and threw them into jail. Those traveling waves would probably go crazy running around the jail cell trying to escape. No matter how hard they tried, though, they'd always end up hitting the jail cell walls. As a result, the poor waves would bounce back and forth and back and forth from one end of the jail cell to the other. Now, if there were several traveling waves trapped in the same jail cell at the same time, one set of waves would end up bouncing off of the left wall, at the same time (and speed) as another set of waves was bouncing off of the right wall. This, of course, is exactly what's required to set up a "standing wave" (two waves traveling in opposite directions at the same speed).
The electron waves that you learned about in the last lesson form standing waves as a result of being trapped inside the atom. What do you think might imprison an electron wave inside an atom? The answer, of course, is that electrons are trapped because they are strongly attracted to the protons in the nucleus. Using the laws of physics to describe the forces of attraction between electrons and protons, scientists can figure out the size and shape of any electron's jail cell. Amazingly, by knowing the size and shape of an electron's jail cell, scientists can tell you what a particular electron standing wave will look like.
Frequently, rather than using words to describe an electron standing wave, scientists use what's known as an electron wave function. Wave functions for electrons, first developed by a man named Erwin Schrödinger, are mathematical expressions that describe the magnitude or "height" of an electron standing wave at every point in space. Now, let's discuss electron energy, which is another important electron property that can be explained and predicted by electron standing waves and their associated wave functions.
Each Wave Function has an Allowed Energy Value
[edit | edit source]Electrons form standing waves whenever they're trapped inside an atom, and thus in order to understand and predict electron behavior, it's important to understand electron standing waves. One of the most important properties that electron standing waves can help to predict is electron energy. The energy of an electron in any atom depends on the size and shape of the electron's standing wave when it's trapped inside that atom. As a result, scientists can use the wave function, or the mathematical description of an electron's standing wave, to figure out how much energy that electron has.
While wave functions are helpful in predicting the amount of energy an electron has, they are also helpful in predicting the amount of energy an electron is allowed to have. In any confined space, like a box, a jail cell, or an atom, only certain standing waves are possible. Why? In order to exist, a standing wave must begin at one side of the box and end at the other. Waves that either don’'t begin where the box begins, or don't end where the box ends aren't allowed. Figure 6.6 shows several allowed standing waves and several forbidden standing waves. Notice that if the wave doesn't "fit" perfectly inside the box, it isn't allowed.

Now here's the really strange thing about describing electrons as standing waves. Since only certain standing waves will fit perfectly inside an atom, electrons trapped in that atom can only have certain electron wave functions with certain electron energies. In other words, the standing wave picture accounts for the fact that some energy values are "allowed" (energy values associated with standing waves that "fit" perfectly inside the atom) while others are "forbidden" energy values (energy values associated with standing waves that do not "fit" perfectly inside the atom). That's exactly what Bohr said when he developed his model to explain atomic spectra! Bohr said that electrons could exist at specific "allowed" energy levels, but that they couldn't exist between those energy levels. Bohr, however, did not have an explanation for why only certain energy levels were allowed. Remarkably, the standing wave description of electrons predicts quantized electron energies just like the Bohr model!
When we represent electrons inside an atom, quantum mechanics requires that the wave must "fit" inside the atom so that the wave meets itself with no overlap; that is, the "electron wave" inside the atom must be a standing wave. If the wave is to be arranged in the form of a circle so that it attaches to itself, the waves can only occur if there is a whole number of waves in the circle.
The standing wave on the left in Figure 6.7 exactly fits in the electron cloud and hence represents an "allowed" energy level whereas the standing wave on the right does not fit in the electron cloud and therefore is not an "allowed" energy level. There are only certain energies (frequencies) for which the wavelength fits exactly to form a standing wave. These are the same energy levels the Bohr model suggested but now there is a reason for why electrons may have only these energies.

Max Born and Probability Patterns
[edit | edit source]There are very few scientists, if any, who can visualize the behavior of an electron during chemical bonding or chemical reactions as standing waves. When chemists are asked to describe the behavior of an electron during a chemical change, they do not describe the mathematical equations of quantum mechanics nor do they discuss standing waves. The behavior of electrons in chemical reactions is best understood in terms of a particle.
Erwin Schrödinger's wave equation for matter waves is similar to known equations for other wave motions in nature. The equation describes how a wave associated with an electron varies in space as the electron moves under various forces. Schrödinger worked out the solutions of his equation for the hydrogen atom and the results agreed with the Bohr values for the energy levels of these atoms. Furthermore, the equation could be applied to more complicated atoms. It was found that Schrödinger's equation gave a correct description of an electron's behavior in almost all cases. In spite of the overwhelming success of the wave equation in describing electron energies, the very meaning of the waves was vague.
A physicist named Max Born was able to attach some physical significance to the mathematics of quantum mechanics. Born used data from Schrödinger's equation to show the probability of finding the electron, as a particle, at the point in space for which Schrödinger's equation was solved. Born's ideas allowed chemists to visualize the results of the wave equation as probability patterns for electron positions.
Suppose we had a camera with such a fast shutter speed that it could take a photo of an electron in an electron cloud and show it frozen in position. We could then take a thousand pictures of this electron at different times and find it in many different positions in the atom. We could then plot all the different electron positions on one picture.
Figure 6.8 shows the result of plotting many different positions of a single electron in the electron cloud of a hydrogen atom. One way of looking at this picture is as an indication of the probability of where you are likely to find the electron in this atom. You must recognize, of course, that the dots are not electrons; this atom has only one electron. The dots are positions where the electron can be found at different times. From this picture, it is clear that the electron spends more time near the nucleus than it does far away. As you move away from the nucleus, the probability of finding the electron becomes less and less. It is also important to note that there is no boundary for this electron cloud. That is, there is no distance from the nucleus where the probability becomes zero.
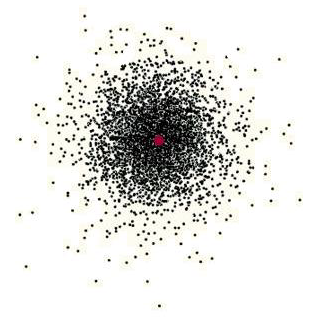
For much of the work we will be doing with atoms, it is convenient to have a boundary for the atom. Most often, chemists choose some distance from the nucleus beyond which the probability of finding the electron becomes very low and arbitrarily draw in a boundary for the atom. Frequently, the boundary is placed such that 90% or 95% of the probability for finding the electron is inside the boundary (Figure 6.9).

Most of the time, we will be looking at drawings of atoms that show an outside boundary for the electron cloud. You should keep in mind, however, that the boundary is there for our convenience and there is no actual boundary on an atom; that is, the probability of finding the electron never becomes zero. This probability plot is very simple because it is for the first electron in an atom. As the atoms become more complicated (more energy levels and more electrons), the probability plots also become more complicated.
Lesson Summary
[edit | edit source]- There are two types of waves – traveling waves that move from one place to another, and standing waves that are stationary. Standing waves are formed when two traveling waves traveling in opposite directions at the same speed combine. Electrons in atoms form standing waves because they are trapped by the attractive forces that exist between their negative charges and the positive charges on the protons in the atom's nucleus. These attractive forces determine the shape and size of the electron's standing wave.
- Mathematical expressions called wave functions are used to describe an electrons standing wave in an atom. The energy of an electron in any atom depends on the size and shape of the electron's standing wave. The wave function can be used to determine the energy of an electron when it is trapped inside an atom.
- Electrons in atoms are only allowed to have certain energy levels (i.e. – those which correspond to standing waves that "fit perfectly" inside the atom). All other electron energies are forbidden. The probability patterns for electrons (electron density) show the probability of finding the electron at a given point.
Review Questions
[edit | edit source]- Choose the correct word in each of the following statements.
- (a) The (more/less) electron density at a given location within the atom the more likely you are to find the electron there.
- (b) If there is no electron density at a particular point in space, there is (no/a high) chance of finding the electron there.
- (c) The higher the probability of finding an electron in a certain spot, the (more/less) electron density there will be at that spot.
- The hydrogen ion, H+ has no electrons. What is the total amount of electron density in a hydrogen atom?
- Decide whether each of the following statements is true or false.
- (a) Only certain electron standing waves are allowed in any particular atom.
- (b) Only certain electron energies are allowed in any particular atom.
- The name for the mathematical expression used to describe an electron standing wave is ________.
- Choose the correct statement.
- (a) Einstein first developed the method of describing electron standing waves with wave functions
- (b) Planck first developed the method of describing electron standing waves with wave functions
- (c) de Broglie first developed the method of describing electron standing waves with wave functions
- (d) Schrödinger first developed the method of describing electron standing waves with wave functions
- Circle all of the statements below which are correct.
- (a) The wave function description of electrons predicts that electrons orbit the nucleus just like planets orbit the sun.
- (b) The wave function description of electrons predicts that electron energies are quantized
- (c) The Bohr model of the atom suggests that electron energies are quantized.
- Fill in the blanks.
- (a) Since only certain values are allowed for the energy of an electron in an atom, we say that electron energies are _________.
- (b) Allowed electron energies correspond to ______________ that fit perfectly in the atom.
- Forbidden electron energies correspond to electron standing waves that ________ in the atom.
Vocabulary
[edit | edit source]- electron density
- The square of the wave function for the electron, it is related to the probability of finding an electron at a particular point in space.
- electron wave function
- A mathematical expression to describe the magnitude, or "height" of an electron standing wave at every point in space.
- standing waves
- Waves that do not travel, or move. They are formed when two traveling waves, moving in opposite directions at the same speed run into each other and combine.
- traveling waves
- Waves that travel, or move.
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License
