High School Chemistry/The Wave Form of Light
Our entire universe is made up of matter, which is anything that has mass and occupies space. You now know that matter is composed of small building blocks known as atoms, and that these small building blocks are composed of even smaller subatomic particles called protons, electrons and neutrons. Matter is all around you and you can use the atomic, or subatomic, description of matter to understand anything from the cells in your body to the planet Earth!
Any object that you can hold or touch is matter. But our universe contains something else – something that you can't really touch, but that you can certainly see (in fact, you can't see without it!), and that you can often feel. It isn't matter, because it doesn't have any mass, nor does it occupy any space. Still it's fundamentally important to our everyday lives, and we most definitely have a name for it. Can you think of what it is? If you haven't guessed by now, the answer is light.
Think about it for a minute – can you really talk about light using any of the ideas that we've considered so far in our study of matter and the universe? Light doesn't have any mass. Light doesn't occupy any space either. Try sticking your hand into the beam of light shining out of a flashlight. Your hand goes straight through as if there was nothing there! And yet there must be something there… how else can you explain the "brightness" that you see? If you have trouble understanding light and trying to define exactly what light is, you're not alone. Scientists had trouble explaining light too. In fact, we've only really understood light for about 100 years.
Lesson Objectives
[edit | edit source]- Define the terms wavelength and frequency with respect to wave-form energy.
- State the relationship between wavelength and frequency with respect to electromagnetic radiation.
- State the respective relationship between wavelengths and frequencies of selected colors on the electromagnetic spectrum.
Wave Form Energy
[edit | edit source]The wave model of energy can be partially demonstrated with waves in a rope. Suppose we tie one end of a rope to a tree and hold the other end at a distance from the tree such that the rope is fully extended.
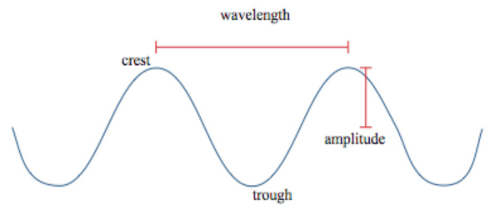
If we then jerk the end of the rope up and down in a rhythmic way, the end of the rope we are holding goes up and down. When the piece of rope we are holding goes up and down, it pulls on the neighboring part of the rope which then also goes up and down. The up and down motion will be passed along to each succeeding part of the rope so that after a short time, the entire rope will contain a wave as shown in Figure 5.1. The red dotted line in the figure shows the undisturbed position of the rope before the wave was initiated. The humps above the undisturbed line are called crests and the dips below the undisturbed position are called troughs. It is important for you to recognize that the individual particles of the rope do not move horizontally. The particles of rope only move up and down and if the wave is allowed to dissipate, all the particles of rope will be in exactly the same position they were in before the wave started. Each hump in the rope moves horizontally from the person to the tree but the particles of rope only move vertically. The energy that is put into the rope by jerking it up and down also moves horizontally from the person to the tree. The feeling that parts of the rope are moving horizontally is a visual illusion.

If we jerk the rope up and down with a different rhythm, the wave in the rope will change its appearance in terms of crest height, distance between crests, and so forth, but the general shape of the wave will remain the same. We can characterize the wave in the rope with a few measurements. An instantaneous photo of the rope will freeze it so we can indicate some of the characteristic values.
The distance from one crest to the next crest is called the wavelength of the wave (Figure 5.2). You could also measure the wavelength from one trough to the next or, in fact, between any two identical positions on successive waves. The symbol used for wavelength is the Greek letter lambda, λ. The distance from the maximum height of a crest to the undisturbed position is called the amplitude of the wave. We could measure a velocity for the wave if we measure how far horizontally a crest travels in a unit of time. The unit for velocity would be the normal meters/second. We also need to determine a very important characteristic of waves called frequency. If we choose an exact position along the path of the wave and count how many crests pass the position per unit time, we would get a value for frequency. In everyday life, frequency values are often expressed as "cycles/second" or "waves/second" but when you try to use these units in calculations, the word "cycles" or "waves" will not cancel. The proper unit for frequency has seconds in the denominator and "1" in the numerator. It is simple 1/s or s−1. This unit has been named "Hertz". Frequencies are often expressed in hertz but when you are plugging numbers into mathematical formulas and wish to keep track of units, it is best to express frequency in units of s−1. The symbol used for frequency is the Greek letter nu, ν. Unfortunately, this Greek letter looks a very great deal like an italicized v. You must be very careful reading equations to be sure whether they are representing velocity, v, or frequency, ν. To avoid this problem, this material will use a lower case f as the symbol for frequency. The velocity, wavelength, and frequency of a wave are related as indicated by the formula, v = fλ. If the wavelength is expressed in meters and the frequency is expressed in s−1, then multiplying the wavelength times the frequency will yield m/s, which is the unit for velocity.
Electromagnetic Waves
[edit | edit source]Electromagnetic radiation (light) waves are somewhat like waves in a rope … except without the rope. Light waves do not need a medium through which to travel. They can travel through a vacuum, which is obvious since they come to us from the sun. The energy of an electromagnetic wave travels in a straight line along the path of the wave just as did the energy in a rope wave. The moving light has associated with it an oscillating electric field and an oscillating magnetic field. This means that along the straight line path of the wave, there exists a positive electric field that will reach maximum positive charge, then slowly collapse to zero charge, and then expand to a maximum negative charge. Along the path of the electromagnetic wave, this changing electric field repeats its oscillating charge over and over again. There is also a changing magnetic field that oscillates from maximum north pole field to maximum south pole field. When scientists try to draw a picture to represent this concept, they use the same picture of a wave that was used for rope waves and water waves.

You should not allow yourself to think that the light travels in this waving pattern. The light travels along the straight red line that represents the undisturbed position. For an electromagnetic wave, the crests and troughs represent oscillating fields, not the path of the light. We can still characterize light waves by their wavelength, frequency, and velocity but these values will be significantly different numerically from water and rope waves.
At some point along the path of the electromagnetic wave, the electric field will reach a maximum value (crest) and then, as the electromagnetic wave continues to move along its straight line path, the electric field will decrease, through zero, increase to a maximum trough, collapse back to zero again, and then expand to another maximum crest. We can measure along the path of the wave, the distance the wave travels between one crest and the succeeding crest and this distance will be the wavelength of the wave. The frequency of electromagnetic waves are determined in the same way as the frequency of a rope wave, that is, the number of full cycles that pass a point in a unit of time. The velocity of electromagnetic waves (in a vacuum) is the same for all waves regardless of frequency or wavelength. Every electromagnetic wave has a velocity of 3.00×108 m/s in a vacuum. The velocity of electromagnetic waves in air is slightly less than in a vacuum but so close that we will use the value for the velocity. The speed of light in a vacuum is symbolized by a lower case c. The relationship for the velocity, wavelength, and frequency of electromagnetic waves is c = λf.
In rope waves and water waves, the amount of energy possessed by the wave is related to the amplitude of the wave; more energy is put into the rope if the end of the rope is jerked higher and lower. But, in electromagnetic radiation, the amount of energy possessed by the wave is related only to the frequency of the wave. In fact, the frequency of an electromagnetic wave can be converted directly to energy in joules by multiplying by a conversion factor. The conversion factor is called Planck's constant and is equal to 6.63×10−34 J·s. Sometimes, the unit for Planck's constant is given as joules/hertz but you can work that out to see the units are the same. The equation for the conversion of frequency to energy is E = hf, where E is the energy in Joules, h is Planck's constant in J·s, and f is the frequency in s−1.
The Electromagnetic Spectrum
[edit | edit source]Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. The highest energy form of electromagnetic waves are gamma (γ) rays and the lowest energy form (that we have named) are radio waves.
On the far left of Figure 5.3 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves. They can also be dangerous to living systems. Humans are advised to limit as much as possible the number of medical x-rays they have per year. Next lower, in energy, are ultraviolet rays. These rays are part of sunlight and the upper end of the ultraviolet range can cause sunburn and perhaps skin cancer. The tiny section next in the spectrum is the visible range of light … this section has been greatly expanded in the bottom half of the figure so it can be discussed in more detail. The visible range of electromagnetic radiation are the frequencies to which the human eye responds. Lower in the spectrum are infrared rays and radio waves.

The light energies that are in the visible range are electromagnetic waves that cause the human eye to respond when those frequencies enter the eye. The eye sends a signal to the brain and the individual "sees" various colors. The highest energy waves in the visible region cause the brain to see violet and as the energy decreases, the colors change to blue, green, yellow, orange, and red. When the energy of the wave is above or below the visible range, the eye does not respond to them. When the eye receives several different frequencies at the same time, the colors are blended by the brain. If all frequencies of light strike the eye together, the brain sees white and if there are no visible frequencies striking the eye, the brain sees black. The objects that you see around you are light absorbers - that is, the chemicals on the surface of the object will absorb certain frequencies and not others. Your eyes detect the frequencies that strike your eye. Therefore, if your friend is wearing a red shirt, it means the dye in that shirt absorbs every frequency except red and the red frequencies are reflected. If your only light source was one exact frequency of blue light and you shined it on a shirt that was red in sunlight, the shirt would appear black because no light would be reflected. The light from fluorescent types of lights do not contain all the frequencies of sunlight and so clothes inside a store may appear to be a slightly different color than when you get them home.
Lesson Summary
[edit | edit source]- One model of light is that of wave-form electromagnetic radiation.
- Light, in wave form, is characterized by its wavelength, λ, frequency, f, and velocity, c.
- The unit for wavelength is meters and the unit for frequency is either s−1 or Hertz.
- The full spectrum of electromagnetic radiation has radio waves as its lowest energy, lowest frequency, longest wavelength end and gamma rays as its highest energy, highest frequency, shortest wavelength end.
- The colors we see for an object are the blending of all the frequencies of light reflected by the object.
Review Questions
[edit | edit source]- Choose the correct word in for the following statement. Blue light has a (longer or shorter) wavelength than red light.
- Choose the correct word in for the following statement. Yellow light has a (higher or lower) frequency than blue light.
- Choose the correct word in for the following statement. Green light has a (larger or smaller) energy than red light.
- If "light A" has a longer wavelength than "light B", then "light A" has _______________ "light B".
- (a) a lower frequency than
- (b) a higher frequency than
- (c) the same frequency as
- If "light C" has a shorter wavelength than "light D", then "light C" has _______________ "light D".
- (a) a larger energy than
- (b) a smaller energy than
- (c) the same energy as
- If "light E" has a higher frequency than "light F", then "light E" has __________________ "light F".
- (a) a longer wavelength than
- (b) a shorter wavelength than
- (c) the same wavelength as
- If "light G" has a higher frequency than "light H", then "light G" has __________________ "light H".
- (a) a larger energy than
- (b) a smaller energy than
- (c) the same energy as
- If "light J" has larger energy than "light K", then "light J" has __________________ "light K".
- (a) a shorter wavelength than
- (b) a longer wavelength than
- (c) the same wavelength as
- Which of the following statements is true?
- (a) The frequency of green light is higher than the frequency of blue light and the wavelength of green light is longer than the wavelength of blue light.
- (b) The frequency of green light is higher than the frequency of blue light and the wavelength of green light is shorter than the wavelength of blue light.
- (c) The frequency of green light is lower than the frequency of blue light and the wavelength of green light is shorter than the wavelength of blue light.
- (d) The frequency of green light is lower than the frequency of blue light and the wavelength of green light is longer than the wavelength of blue light.
- (e) The frequency of green light is the same as the frequency of blue light and the wavelength of green light is shorter than the wavelength of blue light.
- As the wavelength of electromagnetic radiation increases:
- (a) its energy increases.
- (b) its frequency increases.
- (c) its speed increases.
- (d) more than one of the above statements is true.
- (e) none of the above statements is true.
- List three examples of electromagnetic waves.
- Why do white objects appear white?
- Name the colors present in white light in order of increasing frequency.
- Why do objects appear black?
Define these terms: Wavelength: Amplitude: Frequency:
Vocabulary
[edit | edit source]- amplitude of a wave
- The "height" of a wave. In light waves, the amplitude is proportional to the brightness of the wave.
- crest
- High point in a wave pattern (hill).
- electromagnetic spectrum
- A list of all the possible types of light in order of decreasing frequency, or increasing wavelength, or decreasing energy. The electromagnetic spectrum includes gamma rays, X-rays, UV rays, visible light, IR radiation, microwaves, and radio waves.
- frequency of a wave (ν)
- The "number" of waves passing a specific reference point per unit time. The frequency of a light wave determines the color of the light.
- Hertz (Hz)
- The SI unit used to measure frequency. One hertz is equivalent to 1 event (or one full wave passing by) per second.
- trough
- Low point in a wave pattern (valley).
- wavelength (λ)
- The length of a single wave from peak to peak (or trough to trough). The wavelength of a light wave determines the color of the light
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License
