High School Chemistry/Writing Electron Configurations
Lesson Objectives
[edit | edit source]- Figure out how many electrons can exist at any given sublevel.
- Figure out how many different sublevels can exist at any given energy level.
- Be able to write electron configuration of any element given the total number of electrons in that element.
- Be able to write either orbital representations or electron configuration codes.
Electron Configurations
[edit | edit source]How do electrons fill the different energy levels, energy sublevels, and orbitals? To understand that question, it helps a lot to look at a diagram. Figure 7.3 shows the first three energy levels (marked by the large differently colored blocks), and the sublevels (separated by dotted lines) that are present in any atom. In Figure 7.3, each of the circles represents an orbital and, of course, each can hold a total of two electrons. Notice the red n = 1 block contains only an s orbital. That orbital will hold the first two electrons in the atom. Once the red n = 1 block is entirely filled, electrons will start filling the orange n = 2 block. In the orange n = 2 block, there are four different orbitals. The first orbital is an s orbital and the other three are p orbitals. Once all four orbitals in the orange n = 2 block have been filled, electrons will start filling the yellow n = 3 block. In the yellow n = 3 block, there are nine different orbitals. The first orbital is an s orbital, the next three are p orbitals, and the last five are d orbitals. When filling the n = 3 block, the s sublevel will always be filled first, since it is lowest in energy. Again, it can hold at most 2 electrons. After that, the next six will fill the three p orbitals.

So far we've talked about filling the orbitals in Figure 7.3 up to the 3p orbitals. That's a total of 18 electrons. The first 18 electrons are "nice and easy", because they fill the orbitals in order. First, all of the n = 1 orbitals get filled, then all of the n = 2 orbitals get filled, then the n = 3 orbitals get filled. From the 19th electron on, though, things get a little crazy! Before we move on to the that region beyond 18 electrons, let's take a brief look at a shorthand notation that scientists use to signify the electron orbital filling of a given atom.
Since orbitals are filled in order of increasing n and, within each energy level, in order of increasing ℓ, scientists can use a short hand, known as the electron configuration code, to represent filled orbitals. To write the electron configuration code for an atom, you write the symbol for the type of orbital present at a particular sublevel (1s, 2s, 2p, etc.) followed by a superscript to indicate how many electrons are actually in that sublevel in the atom you are describing. Let's take a look at a few examples.
|
Example 1 Nitrogen has 7 electrons. Write the electron configuration for nitrogen. Solution: Take a close look at Figure 7.3, and use it to figure out how many electrons go into each sublevel, and also the order in which the different sublevels get filled. 1. Begin by filling up the 1s sublevel. This gives 1s2. Now all of the orbitals in the red n = 1 block are filled.
2. Next, fill the 2s sublevel. This gives 1s22s2. Now all of the orbitals in the s sublevel of the orange n = 2 block are filled.
3. Notice that we haven't filled the entire n = 2 block yet… there are still the p orbitals!
The overall electron configuration is: 1s22s22p3. |
Overlapping Energy Levels
[edit | edit source]If you had to guess, which orbital do you think would be filled after the 3p orbitals? Most likely you'd guess that the 3d orbitals would come next – and that guess makes a lot of sense. Unfortunately, that guess is also wrong! It turns out that the 4s orbitals are filled before the 3d orbitals, even though the 4s orbitals have n = 4 and the 3d orbitals only have n = 3.
How is that possible? Does it mean that electrons go into higher energy orbitals before completely filling the lower energy orbitals? Is there something wrong with those electrons? Are certain electrons prevented from entering low energy orbitals? What's going on?
It turns out that there's nothing wrong with those 4s electrons at all. They're still behaving like normal electrons and they’re still going into the lowest energy orbital available. The only difference is that the 4s orbital is lower in energy than the 3d orbitals. Sometimes, we get tricked into thinking that the principal quantum number determines which orbitals will get filled first. When it comes to the order in which orbitals are filled, though, the principal quantum number isn't the only factor – what also matters is the energy of the orbital. Usually orbitals with lower principal quantum numbers have lower energies, but that isn't the case when you compare the energies of the 3d orbitals and the energies of the 4s orbitals. In this case, the 4s orbitals are lower in energy than the 3d orbitals even though they have higher principal quantum numbers. Figure 7.4 shows a modified version of Figure 7.3, which shows the n = 4 orbital positions. Let's take a look at an example of an atom where some n = 4 orbitals are filled.

|
Example 2 Potassium has 19 electrons. Write the electron configuration code for potassium. Solution: This time, take a close look at Figure 7.4. 1. Begin by filling up the 1s sublevel. This gives 1s2. Now the n = 1 level is filled.
2. Next, fill the 2s sublevel. This gives 1s22s2
3. Next, fill the 2p sublevel. This gives 1s22s22p6. Now the n = 2 level is filled.
4. Next, fill the 3s sublevel. This gives 1s22s22p63s2
5. Next, fill the 3p sublevel. This gives 1s22s22p63s23p6
Here's where we have to be careful – right after 3p6!!
6. The final electron goes into the 4s sublevel. This gives 1s22s22p63s23p64s1 The overall electron configuration code is: 1s22s22p63s23p64s1. |
The Diagonal Rule
[edit | edit source]Unfortunately,4s orbitals aren't the only ones that get filled earlier than you'd expect based on their principal quantum number. The same turns out to be true of the 5s orbitals as well. Even though 5s orbitals have a higher principal quantum number than 4d orbitals, (n = 5 compared to n = 4), they're actually lower in energy. As a result, 5s orbitals are always filled before 4d orbitals. Similarly, 6s orbitals are lower in energy than 5d orbitals, so 6s orbitals are always filled first. The story gets even stranger when you consider f orbitals. 5s, 5p, and 6s orbitals are all lower than 4f orbitals. In other words, before you can get an electron into a 4f orbital, you must first fill up the 5s orbitals, the 5p orbitals and the 6s orbitals.
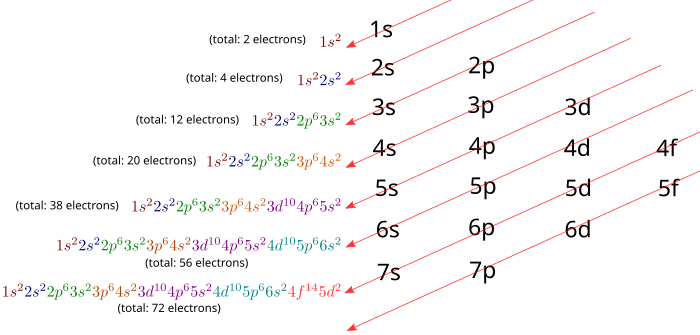
Filling up orbitals and writing electron configurations was so easy for atoms with less than 18 electrons! But for atoms with more than 18 electrons, it seems hopeless to memorize all of the different rules. How will you ever get straight whether the 5s orbital is higher or lower than the 4d orbital or the 4f orbital? Thankfully, there's a simple rule known as the diagonal rule. The diagonal rule states that: Electrons fill orbitals in order of increasing "quantum number sum" (n + ℓ). When two orbitals share the same quantum number sum, they will be filled in order of increasing n.
Luckily, the diagonal rule also has a diagram that's easy to remember, and that allows you to easily figure out the order in which electron orbitals are filled. The below figure shows the diagram. In order to use it, you must follow the arrows from tail-to-tip, starting with the first arrow in the upper left-hand corner, and working your way down through the arrows to the lower right-hand corner of the diagram. To see how this works, let's take a look at an example.

|
Example 3 Hafnium has 72 electrons. Write the electron configuration for hafnium. Solution: We can follow the arrows in the diagram (as shown below), until we have assigned all 72 electrons. Notice how you fill orbitals as you progress along the arrows, starting from the top arrow and moving down. Remember to stop once you hit 72. At that point, you have finished writing the electron configuration for Hafnium. |
Now that we know how electrons are assigned to orbitals, we can start to talk about how different orbitals, and different electron configurations actually affect the chemical properties of different atoms. In other words, we can actually start to talk about chemistry! Sometimes it seems that quantum physics and quantum chemistry are very far removed from the real world chemistry that we see and use in every day life. How are orbitals important when it comes to developing new drugs to treat cancer? How are energy levels important when it comes to inventing different kinds of superconductors or plastics? How are electron standing waves important when it comes to testing for toxins in your food? All of these processes are determined by chemical properties which are, themselves, a direct result of how electrons are arranged, and interact within different atoms and molecules. Sometimes it is easy to forget about the electrons, and some of the strange quantum properties of subatomic particles. Nevertheless, we will never be able to fully understand chemistry, if we don't understand its smallest components – components like electrons, protons, neutrons and, of course, the atom.
Lesson Summary
[edit | edit source]- For any atom with less than 18 electrons, orbitals are filled in order of increasing n and, for any given n, in order of increasing ℓ.
- Electron configurations are a shorthand notation for representing the filled orbitals in a given atom. They are written using the principal quantum number, n, for the energy level, the letter (s, p, d, or f) for the sublevel, and a superscript for the number of electrons in that sublevel.
- For atoms with more than 18 electrons, the orbitals are filled in order of increasing n (and in order of increasing ℓ for a given n) up to the 18th electron; however after the 18th electron the 4s orbital are filled before the 3d orbitals. This is because the 4s orbital has lower energy than the 3d orbital.
- The diagonal rule states that electrons fill orbitals in order of increasing "quantum number sum" (n + ℓ). When two orbitals share the same "quantum number sum", they will be filled in order of increasing n.
Review Questions
[edit | edit source]- Write the electron configuration for beryllium. Beryllium has 4 electrons.
- Write the electron configuration for silicon. Silicon has 14 electrons.
- Write the electron configuration for nitrogen. Nitrogen has 7 electrons.
- Write the electron configuration for chromium. Chromium has 24 electrons.
- Write the electron configuration for silver. Silver has 47 electrons.
Vocabulary
[edit | edit source]- diagonal rule
- The electrons fill orbitals in order of increasing "quantum number sum" (n + ℓ). When two orbitals share the same "quantum number sum", they will be filled in order of increasing n.
- electron configuration
- A short hand notation to indicate the electron orbitals which are filled in a particular atom.
- quantum number sum
- The sum of the principal quantum number, n, and the azimuthal quantum number, ℓ, for an electron. That is n + ℓ.
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License