High School Earth Science/Identification of Minerals
Suppose you bought a new shirt with the money you saved from your allowance. How would you describe your shirt when you are talking to your best friend on the phone? You might describe the color, the way the fabric feels, and the length of the sleeves. These are all physical properties of your shirt. If you did a good job describing your shirt, your friend would recognize the shirt when you wear it. Minerals also have physical properties that are used to identify them.
Lesson Objectives
[edit | edit source]- Explain how minerals are identified.
- Describe how color, luster, and streak are used to identify minerals.
- Explain how the hardness of a mineral is measured.
- Describe the properties of cleavage and fracture.
- Identify additional properties that can be used to identify some minerals.
How are Minerals Identified?
[edit | edit source]Imagine you were given a mineral sample similar to the one shown in Figure 3.12. How would you try to identify your mineral? If you were a mineralogist, you would use certain properties to identify the mineral. You can observe some properties by looking at the mineral. For example, you can see that the mineral in Figure 3.12 is the color of gold and is shiny. But, you cannot see all mineral properties. You need to do simple tests to determine some properties, such as how hard the mineral is. You can use a mineral's properties to determine its identity because the properties are determined by the chemical composition and crystal structure, or the way that the atoms are arranged.

Color
[edit | edit source]Color is probably the easiest property to observe. Unfortunately, you can rarely identify a mineral only by its color. Sometimes different minerals are the same color. Take another look at Figure 3.12. The mineral is a gold color, so you might think that it is gold. The mineral is actually pyrite, or "fool's gold", which is made of iron and sulfide. It contains no gold atoms.
Often, the same mineral comes in different colors. Figure 3.13 shows two samples of quartz—one is colorless (although on a purple background) and one is purple. The purple color of the quartz comes from a tiny amount of iron in the crystal. The iron in quartz is a chemical impurity because it is not normally found in quartz. Many minerals are colored by chemical impurities. Other factors, such as weathering, can also affect a mineral’s color. Weathering affects the surface of a mineral. Because color alone is unreliable, geologists identify minerals by several traits.
Streak
[edit | edit source]
Streak is the color of the powder of a mineral. To do a streak test, you scrape the mineral across an unglazed porcelain plate. The plate is harder than many minerals, causing the minerals to leave a streak of powder on the plate. The color of the streak often differs from the color of the larger mineral sample, as Figure 3.14 shows. If you did a streak test on the yellow-gold pyrite, you would see a blackish streak. This blackish streak tells you that the mineral is not gold because gold has a gold-colored streak.
Streak is a more reliable property than the color of the mineral sample. The color of a mineral may vary, but its streak does not vary. Also, different minerals may be the same color, but they may have a different color streak. For example, samples of hematite and galena can both be dark gray, but hematite has a red streak and galena has a gray streak.
Luster
[edit | edit source]Luster describes the way light reflects off of the surface of the mineral. You might describe diamonds as sparkly or pyrite as shiny, but mineralogists have special terms to describe the luster of a mineral. They first divide minerals into metallic and non-metallic luster. Minerals like pyrite that are opaque and shiny have a metallic luster. Minerals with a non-metallic luster do not look like metals. There are many types of non-metallic luster, six of which are described in Table 3.1.
| Non-Metallic Luster | Appearance |
|---|---|
| Adamantine | Sparkly |
| Earthy | Dry, clay-like |
| Pearly | Pearl-like |
| Resinous | Like resins, such as tree sap |
| Silky | Soft-looking with long fibers |
| Vitreous | Glassy |
Can you match the minerals in Figure 3.15 (below) with the correct luster from Table 3.1 (above) without looking at the caption?
Density
[edit | edit source]You are going to visit a friend. You fill one backpack with books so you can study later. You stuff your pillow into another backpack that is the same size. Which backpack will be easier to carry? Even though the backpacks are the same size, the bag that contains your books is going to be much heavier. It has a greater density than the backpack with your pillow.
Density describes how much matter is in a certain amount of space. Substances that have more matter packed into a given space have higher densities. The water in a drinking glass has the same density as the water in a bathtub or swimming pool. All substances have characteristic densities, which does not depend on how much of a substance you have.
Mass is a measure of the amount of matter in an object. The amount of space an object takes up is described by its volume. So, density of an object depends on its mass and its volume. Density can be calculated using the following equation.
Samples that are the same size, but have different densities, will have different masses. Gold has a density of about 19 g/cm3. Pyrite has a density of only about 5 g/cm3. Quartz is even less dense than pyrite and has a density of 2.7 g/cm3. If you picked up a piece of pyrite and a piece of quartz that were the same size, the pyrite would seem almost twice as heavy as the quartz.
Hardness
[edit | edit source]| Hardness | Mineral |
|---|---|
| 1 | Talc |
| 2 | Gypsum |
| 3 | Calcite |
| 4 | Fluorite |
| 5 | Apatite |
| 6 | Orthoclase feldspar |
| 7 | Quartz |
| 8 | Topaz |
| 9 | Corundum |
| 10 | Diamond |
Hardness is a mineral's ability to resist being scratched. Minerals that are not easily scratched are hard. You test the hardness of a mineral by scratching its surface with a mineral of a known hardness. Mineralogists use Mohs Scale, shown in Table 3.2 (above), as a reference for mineral hardness. The scale lists common minerals in order of their relative hardness. You can use the minerals in the scale to test the hardness of an unknown mineral.
As you can see, diamond is a 10 on Mohs Scale. Diamond is the hardest mineral, which means that no other mineral can scratch a diamond. Quartz is a 7, so it can be scratched by topaz, corundum, and diamond. Quartz will scratch minerals, such as fluorite, that have a lower number on the scale. Suppose you tested a piece of pure gold for hardness. Calcite would scratch the gold, but gypsum would not because gypsum is a 2 and calcite is a 3. That would mean gold is between the hardness of gypsum and calcite, or 2.5 on the scale. A hardness of 2.5 means that gold is a relatively soft mineral. It is only about as hard as your fingernail.
Cleavage and Fracture
[edit | edit source]Minerals break apart in characteristic ways. Remember that all minerals are crystalline, which means that the atoms in a mineral are arranged in a repeating pattern. The pattern of atoms in a mineral determines how a mineral will break. When you break a mineral, you break chemical bonds. Because of the way the atoms are arranged, some bonds are weaker than other bonds. A mineral is more likely to break where the bonds between the atoms are weaker.
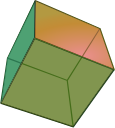
Cleavage is the tendency of a mineral to break along certain planes to make smooth surfaces. Minerals with different crystal structures will cleave in different ways, as Figure 3.16 shows(below). Halite tends to form cubes with smooth surfaces, mica tends to form sheets, and fluorite can form octahedra.
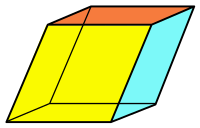
Minerals can form various shapes like the polygons, shown in Figure 3.17, when they are broken along their cleavage planes. The cleavage planes are important for people who cut gemstones, such as diamonds and emeralds. The planes determine how the crystals can be cut to make smooth surfaces.
Fracture describes how a mineral breaks when it is not broken along a cleavage plane. All minerals break but fracture describes a break when the resulting surface is not smooth and flat. You can learn about a mineral from the way it fractures. Jagged edges are usually formed when metals break. If a mineral splinters like wood it may be fibrous. Some minerals, such as quartz, form smooth curved surfaces when they fracture. A mineral that broke forming a smooth, curved surface is shown in Figure 3.18 (below).

Other Identifying Characteristics
[edit | edit source]Minerals have some other properties that can be used to identify them. For example, a mineral's crystal structure can be used to help identify the mineral. Sometimes, a trained mineralogist can tell the crystal structure just by looking at the shape of the mineral. In other cases, the crystals in the mineral are too small to see and a mineralogist will use a special instrument that uses X rays to find out the crystal structure.
Some unusual and interesting properties can be used to identify certain minerals. Some of these properties are listed in Table 3.3. Although these properties are rare, several minerals have them. An example of a mineral that has each property is also listed in Table 3.3.
| Property | Description | Example of Mineral |
|---|---|---|
| Fluorescence | Mineral glows under ultraviolet light | Fluorite |
| Magnetism | Mineral is attracted to a magnet | Magnetite |
| Radioactivity | Mineral gives off radiation that can be measured with Geiger counter | Uraninite |
| Reactivity | Bubbles form when mineral is exposed to a weak acid | Calcite |
| Smell | Some minerals have a distinctive smell | Sulfur (smells like rotten eggs) |
Lesson Summary
[edit | edit source]- You can identify a mineral by its appearance and other properties.
- The color and luster describe the appearance of a mineral, and streak describes the color of the powdered mineral.
- A mineral has a characteristic density.
- Mohs hardness scale is used to compare the hardness of minerals.
- The way a mineral cleaves or fractures depends on the crystal structure of the mineral.
- Some minerals have special properties that can be used to help identify the mineral.
Review Questions
[edit | edit source]- Which properties of a mineral describe the way it breaks apart?
- A mineral looks dry and chalky. Why sort of luster does it have?
- You are trying to identify a mineral sample. Apatite scratches the surface of the mineral. Which mineral would you use next to test the mineral's hardness—fluorite or feldspar? Explain your reasoning.
- Why is streak more reliable than color when identifying a mineral?
- You have two mineral samples that are about the size of a golf ball. Mineral A has a density of 5 g/cm3. Mineral B is twice as dense as Mineral A. What is the density of Mineral B?
- Why do some minerals cleave along certain planes?
Vocabulary
[edit | edit source]- cleavage
- The tendency of a mineral to break along certain planes to make smooth surfaces.
- density
- How much matter is in a certain amount of space; mass divided by volume.
- fracture
- The way a mineral breaks when it is not broken along a cleavage plane.
- hardness
- The ability to resist scratching.
- luster
- The way light reflects off of the surface of the mineral.
- streak
- The color of the powder of a mineral.
Points to Consider
[edit | edit source]- Some minerals are colored because they contain chemical impurities. How did the impurities get into the mineral?
- What two properties of a mineral sample would you have to measure to calculate its density?