Mathematics for Chemistry/Trigonometry
Trigonometry - the sin and cosine rules
[edit | edit source]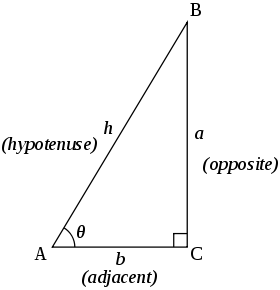
In the following trigonometric identities, , and are the lengths of sides in a triangle, opposite the corresponding angles , and .
- The sin rule
- The cosine rule
- The ratio identity
Trigonometric identities
[edit | edit source]
Remember this is a consequence of Pythagoras' theorem where the length of the hypotenuse is 1.
The difference of two angles can easily be generated by putting and remembering and . Similarly, the double angle formulae are generated by induction. is a little more complicated but can be generated if you can handle the fractions! The proofs are in many textbooks but as a chemist it is not necessary to know them, only the results.
Identities and equations
[edit | edit source]Identities and equations look very similar, two things connected by an equals sign. An identity however is a memory aid of a mathematical equivalence and can be proved. An equation represents new information about a situation and can be solved.
For instance,
is an identity. It cannot be solved for ; it is valid for all . However,
is an equation where .
If you try and solve an identity as an equation you will go round and round in circles getting nowhere, but it would be possible to dress up into a very complicated expression which you could mistake for an equation.
Some observations on triangles
[edit | edit source]Check you are familiar with your elementary geometry. Remember from your GCSE maths the properties of equilateral and iscoceles triangles. If you have an iscoceles triangle you can always dispense with the sin and cosine rules, drop a perpendicular down to the base and use trig directly. Remember that the bisector of a side or an angle to a vertex cuts the triangle in two by area, angle and length. This can be demonstrated by drawing an obtuse triangle and seeing that the areas are .
The interior angles of a polygon
[edit | edit source]Remember that the interior angles of a -sided polygon are (in degrees) or () (in radians)
For benzene there are six equilateral triangles if the centre of the ring is used as a vertex, each of which having an interior angle of 120 degrees. Work out the angles in azulene, (a hydrocarbon with a five and a seven membered ring), assuming all the C-C bond lengths are equal, (this is only approximately true).























