Medical Physiology/Basic Biochemistry/Amino Acids and Proteins
Proteins are large molecules made from smaller building blocks called amino acids. If there are less than 10 amino acids the molecule is called a peptide; if 10 to 100 a polypeptide; and more than 100 a 'protein. There are twenty different amino acids, and the body can synthesize eleven of them. The other nine must be obtained in the diet, and are known as the essential amino acids.
Amino Acid Structure
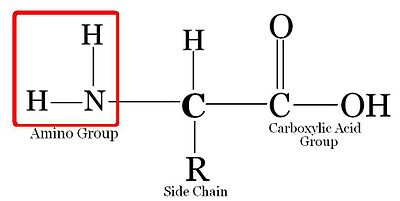
[edit | edit source]Amino acids all have the basic backbone. They all consist of a carbon atom (C) attached to a carboxyl group (-COOH), an amino group, (-NH2), a Hydrogen, and another group of atoms (R). The R group gives the amino acid its unique characteristics, and allows it to react with other amino acids in unique ways. Every protein starts with Methionine (Met). Met attaches to the N-terminal end (amine group). That amine group attaches to carboxylic group of the next amino acid. That carboxylic group then attaches to amine group of the next amino acid. Here is a depiction of a 'model' amino acid: amino acid is a basic unit of protein.
And here is a group of typical amino acids. Note the sulphur atom in methionine:
The Amino Acids
[edit | edit source]Below are the names of the 20 amino acids. Each amino acid has a three letter designation as well as a single letter designation. The amino acids are sorted in eight groups aliphatic, organic acid, amide, organic base, sulfur containing, alcohol containing, imine, and aromatic. Aliphatic (carbon side chains) groups consists of alanine, glycine, valine, leucine and isoleucine. The organic acid group consist of glutamic and aspartic acid. With a similar structure to the acids, the amides are aspargine and glutamine. The group of organic base consist of arginine, lysine, and histidine. Those amino acids consisting of sulfur are methionine and cysteine. Proline is the only imine. The alcohols are serine, threonine, and tyrosine (also an aromatic) and the aromatic amino acids consists of tryptophan and phenylalanine.
| Amino Acid | Three Letter Abbreviation |
Single Letter Symbol |
|---|---|---|
| Alanine | Ala | A |
| Arginine | Arg | R |
| Asparagine | Asn | N |
| Aspartate or Aspartic Acid | Asp | D |
| Cysteine | Cys | C |
| Glutamic Acid | Glu | E |
| Glutamine | Gln | Q |
| Glutamine or Glutamic Acid | Glx | Z |
| Glycine | Gly | G |
| Histidine* | His | H |
| Isoleucine* | Ile | I |
| Leucine* | Leu | L |
| Lysine* | Lys | K |
| Methionine* | Met | M |
| Phenylalanine* | Phe | F |
| Proline | Pro | P |
| Serine | Ser | S |
| Threonine* | Thr | T |
| Tryptophan* | Trp | W |
| Tyrosine | Tyr | Y |
| Valine* | Val | V |
Asterix [*] denotes essential amino acid.
Classification of Amino Acids
[edit | edit source]Although there are many ways to classify amino acids, these molecules can be assorted into 6 main groups, on the basis of their structure and general chemical characteristics of their R groups.
| Classes of Amino Acids | Name of the Amino Acid |
|---|---|
| Aliphatic | Glycine, Alanine, Valine, Leucine, Isoleucine |
| Hydroxyl or Sulfur-containing |
Serine, Cysteine, Threonine, Methionine |
| Cyclic | Proline |
| Aromatic | Phenylalanine, Tyrosine, Tryptophan |
| Basic | Histidine, Lysine, Arginine |
| Acidic and their amide |
Aspartate, Glutamate, Asparagine, Glutamine |
Peptide Structure
[edit | edit source]When two amino acids join together the amino group of one links with the acid group of the other, with the loss of a molecule of water. Amino acids are linked by peptide bond.

Protein Structure
[edit | edit source]
Because of their bonding proteins will take on numerous different forms and shapes. Proteins have what is called a primary, a secondary, a tertiary, and a quaternary structure. The primary structure describes the sequencing of the amino acid. It is very useful to know the amino acid sequence of a protein. Some of the uses of knowing amino acid sequences of proteins are following: similarity among proteins, evolution of proteins, existence of repeats or modules, identify localization signals, prepare antibodies against a protein, and allow the design of DNA probes to locate a gene. The secondary structure describes the special arrangement of the amino acids in the chain and is usually either helical in nature, or so called beta-pleated; and the tertiary structure describes the three dimensional shape of the protein and is usually either fibrous or globular. The quaternary structure is the specific association of multiple polypeptide chains to form multisubunit complexes.
More can be found on [protein structure] at Wikipedia.
The Secondary Structure
[edit | edit source]The secondary structure of amino acids consists of two major components: alpha helix and beta strands. The alpha helix consist of various hydrogen bonds to the nitrogen and oxygen components in a n + 4 matter. A looser or tighter alpha helices also exist: n + 5 or n + 3. On the other hand, beta strands do not form helices: they form sheets. Beta sheets can be displayed in two different ways: parallel and antiparallel. As the title states, the parallel beta sheets run perfectly on top of each other, whereas antiparallel beta sheets alter in their direction. Even though it may sound as the parallel are more stable, the truth is the antiparallel are more stable. The beta sheets can be seen in the following weblink[[1]]
References
[edit | edit source]Williams, R. M. Amino Acids. Kidlington: Elsevier, 2008. Print. Berg, Jeremy M., John L. Tymoczko, and Lubert Stryer. Biochemistry. 7th ed. New York: W.H. Freeman, 2012. Print. Branden, Carl, and Tooze, John. Introduction to Protein Structure. New York: Garland Publishing, 1999. Print. Wissmann, Paul. Amino Acids and Proteins. Web. Oct. 28, 2012. http://homepage.smc.edu/wissmann_paul/anatomy2textbook/AAcidsProteins.html