Medical Physiology/Basic Biochemistry/Introduction and Overview
A knowledge of the basics of biochemistry is important for the understanding of physiology. This section acts as an introduction to biochemistry, and is really the minimum that you need to understand.
The four main biochemical groups are Sugars, Fats, Amino Acids, and Nucleotides. We will look briefly at each of these in turn and see how they play their roll in energy storage, energy production, protein synthesis, & cell reproduction. We will also look at how energy is utilized by the cell.
Sugars
[edit | edit source]The most important source of energy for cells is the mono-saccharide sugar glucose which has the general formula C6H1206. This is broken down in the presence of oxygen to produce energy, carbon dioxide and water:
C6H1206 + 6O2 --->6H20 + 6CO2 + Energy (36-38 mols of ATP from ADP).
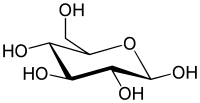
Glucose is taken into the body as various sugars and complex carbohydrates (starches). The enzymes in the digestive process and various cells produces Glucose. In the body glucose can be built up into a starch Glycogen which can be stored in the liver and in muscle. This is the body's reserve supply of glucose. Energy can be also obtained from Proteins and Fats, but Glucose is particularly important, because it is the only source of energy for the brain cells and if the brain is without glucose for a period of 6-8 minutes, brain death will occur. Here is the structure of Glucose. It is usually represented as the first image, or simply a hexagonal ring.
Glucose is broken up in a two step process. First it is broken down into two molecules of pyruvate by a process called glycolosis. This occurs in the cytoplasm of the cell. Then if oxygen is present, the pyruvate is taken into the mitochondria, and is broken down into Acetyl CoA which enters the citric acid cycle, producing high energy hydrogen bonds. The mitochondria will process these bonds into high energy ATP (Adenosine tri-phosphate) from ADP (Adenosine di-phosphate). This ATP is used to power the reactions of the cell. Some 36-38 molecules of ATP are produced for each glucose molecule so processed.This process is shown graphically in the following flow sheet.
In the absence of oxygen (such as may occur in severe exercise), the pyruvate will be processed into lactic acid (anaerobic tissue respiration), and only two molecules of ATP will be produced. In mammals this is insufficient for the body's needs, and in the absence of oxygen cell death will occur in most tissues.
See Sugars for more details.
Fats
[edit | edit source]Fats or lipids are the main way that the body stores excess nutrients. One Gram of fat will produce about 9 Kilocals, as opposed to 4 Kilocals for carbohydrate or protein.
Fats consist of 1-3 fatty acids attached to a glycerol molecule.
-IMAGE
Fatty acids consist of the general formular R-COOH, where R is a carbohydrate chain.
-IMAGE
Fatty Acids can be metabolized in almost all cells of the body, the notable exception are brain cells, to produce ATP from ADP. They are broken down by a process called beta-oxidation to produce two carbon units which are then fed into the Citric Acid Cycle via Acetyl CoA.
Other important lipid related compounds are Cholesterol, important in its own right and essential as a precursor for Steroids, and phospholipids which form the walls of all cell membranes.
See Fats for more details.
Amino Acids & Proteins
[edit | edit source]Proteins are made from several amino acids strung together as a chain. They are important for the structural elements of the body, either as intracellular components, or as the substrate of body tissues. Most enzymes and hormones are also proteins. Their versatility is due to the many shapes that they can take up.
Overview
[edit | edit source]Proteins are made from several amino acids strung together as a chain. They are important for the structural elements of the body, either as intracellular components, or as the substrate of body tissues. Most enzymes and hormones are also proteins. Their versatility is due to the many shapes that they can take up.
Amino Acid
[edit | edit source]A typical amino acid consists of a carbon atom attached to a carboxyl group -COOH and an amino -NH2 group. Below is shown the general model, and the isoleucine amino acid as an example.
-IMAGE
Amino acids are strung together to form peptides - less than 100 amino acids - and proteins - more than 100 amino acids. Peptides are assembled on RNA templates.
Protein Synthesis
[edit | edit source]An RNA template is made from the DNA, and after processing this leaves the nucleus and is attached to a ribosome. The following illustrates this process
-IMAGE
After becoming attached to the ribosome, Transfer RNA molecules will attach to individual amino acids and transfer them on to the RNA template:
-IMAGE
The attachment is formed by the removal of a molecule of water between the carboxyl and the amino group:
-IMAGE
As well as being important for structural elements, amino acids are an important source for the manufacture of Glucose in the starvation state.
Protein Metabolism
[edit | edit source]If Glycogen supplies are exhausted, glucose can be made from amino acids by a process called gluconeogenesis. The amino acid is de-aminated, and the resulting organic acid will be utilized either in the Glycolosis pathway or the Citric Acid Cycle to make Glucose.
-IMAGE
See Amino Acids & Proteins for more details.
Nucleic Acids
[edit | edit source]See Nucleic Acids for more details.
Enzymes
[edit | edit source]See Enzymes for more details.