Planet Earth/2b. Solar Energy
A Fallen Scientist
[edit | edit source]
On a cold late November day in 1997, Fred Hoyle found himself injured, with his body smashed against tomb-like granitic rocks at the base of a large cliff near Shipley Glen in central England. His shoulder bones were broken, his kidneys malfunctioning, and blood dripped from his head. He could not move, on the edge of death. He had no notion how long he had been laying at the base of the cliff, as it was dark, the moss-covered stones and cragged tree branches hovered over him in the dim light. But then it came. The sun. It illuminated the sky, burning bright, and he remembered who he was.

Fred Hoyle was a physicist of the sun. Fifty years before, he wrote his greatest works, a series of scientific papers published between 1946 and 1957, and in the process discovered how the sun generates its energy through the generation of mass, in particular the generation of new forms of atoms of differing masses. He had founded a new field of science called stellar nucleosynthesis. In the early years of the twentieth century, scientists had discovered that enormous amounts of energy could be released with the spontaneous decay of radioactive atoms. This loss of atomic mass, is called nuclear fission, and by the 1940s, this power was harnessed in the development of atomic weapons and nuclear power. The sun, however, emits its energy due to nuclear fusion, the generation of atoms with increasing atomic mass. Fred Hoyle was at the forefront of this research, as he suggested that all atoms of the universe, are formed initially in stars, such as the sun.
Above him, the injured Fred Hoyle observed the sun. Its bright light illuminating the morning sky, glowing a yellowish white. It is a giant of the solar system, 1.3 million planets the size of Earth could fit inside the volume of the sun. It has a mass 333,000 times larger than the Earth. It is beyond the imagination of large, and while stars elsewhere in the universe dwarf the sun, its enormous size is nearly incomprehensible.
Stars are classified by their color (which is related to temperature), and luminosity (or brightness), which is related to the size of the star. The sun sits in the center of a large pack of stars called the Main Sequence, in a diagram plotting color and luminosity called the Hertzsprung–Russell diagram. The sun’s yellowish-white light spectrum indicates an average surface temperature of 5,778 Kelvin, and luminosity of 1 solar unit. Along the main sequence of stars, each star can be grouped by their color.

Annie Jump Cannon developed what is called the Harvard system, which uses letters to denote different colors, which relates to temperature. Using this system, the sun is a class G-star. The hottest blue stars are class O, with the cooler red stars class M. The series was used to map the night sky, denoting stars following a sequence from hottest to coolest (O, B, A, F, G, K, and M). O and B stars tend to be blueish in color, while A and F stars tend to be white in color, G are more yellow, while K and M are pink to red in color. 90% of stars fit on this Main Sequence of stars, however some odd-balls lay outside of this Main Sequence, including, the highly luminous Giant Stars (Supergiants, Bright Giants, Giants and Subgiants), and lower luminous White Dwarfs.
The Anatomy of the Sun
[edit | edit source]
The outer crown of the sun is its atmosphere, composed of a gaseous halo seen when the Sun is obscured by the Moon during a solar eclipse. As a highly dynamic layer, giant flares erupt from this region of the Sun. The Corona is an aura of plasma (composed of highly charged free electrons) much like lightning bolts, which reach far into the space around the Sun. Solar Prominences are loop-like features that rise 800,000 kilometers above the Sun, and Solar Flares, arise from the margins of dark Sun Spots. Sun Spots are cooler regions of the Sun’s Photosphere, which are a few thousand Kelvin cooler than the surrounding gas. These Sun Spots have been observed for hundreds of years, and follow an 11-year cycle, related to the Sun’s magnetic orbit around its core. Sun spots appear just above and below the Sun’s equator in a clear 11-year burst of activity. Sun flares during this enhanced Sun Spot activity result in charge particles hitting the outer most atmosphere of Earth, resulting in a colorful Aurora in the night sky near the Earth’s magnetic poles during these events. Sun spot activity is closely monitored, since it can affect Earth orbiting satellites. (see http://www.solarham.net/). Measurements of solar irradiance hitting the upper atmosphere, measured by NASA satellites Nimbus 7 (launched in 1978) and Solar Maximum Mission (launched in 1980), among others since then, show slight increases of total solar irradiance striking the upper atmosphere during periods of sun spot activity. This is due to faculae, which are brighter regions that accompany sun spot activity, giving an overall increase of solar irradiance, however when darker sun spots dominate over the brighter faculae regions there has also been brief downward swings of lower solar irradiance during sun spot activity as well. Measured solar irradiance of the upper atmosphere since these satellites were launched in 1978 has shown that the sun’s energy striking the Earth’s upper atmosphere only varies between 1369 and 1364 watts/meter2 during these events (Willson & Mordvinov, 2003: Geophyscial Research Letters).

Temperatures are highest in the upper atmosphere of the sun, since these blasts of plasma excite the free particles producing temperatures above 1 million Kelvin. The lowest level of the Sun’s atmosphere is the Transition Zone, which can bulge upward through convection. Convection is the movement of energy along with matter which can effectively transport energy from the inner portion of the Sun upward. Below the Transition Zone is the much thicker Chromosphere, where the temperature is around 5,778 Kelvin. The Chromosphere is the red color seen only during Solar eclipses. Below the Chromosphere is the Photosphere, which unlike the upper atmospheric layers of the Sun is held by Sun’s gravity and represents more dense matter. Although the Sun does not have a clearly defined surface, the top of the denser Photosphere can be viewed as the “surface” of the sun, since it is made of more dense matter. Below the Photosphere are two zones which transport energy outward from the Core of the Sun. The upper zone is the Convection Zone, in which Energy is transferred by the motion of matter (Convection), while the lower zone is the Radiative Zone, in which Energy is transferred without the motion of matter, called Conduction. Between these two zones is the Tachocline. The inner Core of the Sun, representing about 25% of the Sun’s inner radius, is under intense gravitational force, enough to result in nuclear fusion.
The Sun’s Nuclear Fusion Reactor
[edit | edit source]The Sun’s energy is generated from the intense gravity crushing particles called protons into neutrons. When two protons are crushed together in the core of the sun, overcoming the electro-magnetic force that normally repels them, one proton will release sub-atomic particles and convert to a neutron. The result of this change from proton to neutron releases energy, as well as a positron and neutrino. The Earth is bombarded every second with billions of these solar neutrinos, which pass through matter unimpeded and often undetected, since they are neutrally changed and have an insufficient amount of mass to interact with other particles. Released positrons make their way upward from the core of the sun and interact with electrons which encircle the sun, and are annihilated when each positron comes in contact with an electron. Electrons, which are abundant as negatively charged plasma of the extremely hot outer layers of the sun, prevent positrons from reaching the Earth.
In chemistry, the simplest atom is just a single negatively charged electron surrounded by a single positively charged proton, what chemist’s call hydrogen. With an addition of a neutron, the atom turns from being hydrogen, to something called deuterium, which is an isotope of hydrogen. Deuterium carries double the atomic mass of hydrogen, since each proton and neutron carries an atomic mass of 1, so deuterium has a mass of 2. While electrons, neutrinos and positrons have an insignificant amount of mass, or a mass close to zero.

The incredible gravitational force of the sun, breaks apart atoms, with electrons pushed upward away from the core of the sun, forming a plasma of free electrons in outer layers of the sun, while leaving protons within the center of the sun. Electrons, which carry a negative charge are attracted to Protons, which carry a positive charge. Outside of the gravity of the sun, the free Protons and Electrons would attract each other to form the simplest atom, the element Hydrogen. Inside the core of the sun, however, the Protons are crushed together forming Neutrons. This process is called the Proton–Proton chain reaction. There is some debate on how protons in the core of the Sun are crushed together, recent experiments suggest that protons are brought so close together they form diprotons, where two protons come together forming a highly unstable isotope of Helium. Elements are named based on the number of protons they contain, for example Hydrogen contains 1 proton, while Helium contains 2 protons. During this process protons are also converted to Neutrons. The addition of Neutrons helps destabilize atoms of Helium, that contain 2 protons. The Proton–Proton chain reaction within the sun takes free Protons, and converts them through a process of steps into atoms of Helium, which contain 2 Protons and 2 Neutrons. Elements with differing numbers of Neutrons are called Isotopes, hence, inside the core of the sun are the following types of atoms:
- 1 proton + 0 neutrons (Hydrogen)
- 2 protons + 0 neutrons (Helium‑2 isotope)
- 1 proton + 1 neutron (Hydrogen‑2 isotope, Deuterium)
- 2 protons + 1 neutron (Helium‑3 isotope)
- 2 protons + 2 neutrons (Helium‑4 isotope)
Through this process, Hydrogen with a single Proton is converted to Helium‑4 with two Protons and two Neutrons, resulting in larger atoms inside the core of the Sun over time.
How the Sun Made the Larger Elements
[edit | edit source]The proposed idea for this proton-proton chain reaction within the sun’s core was first theorized by a group of physicists in 1938, working together to solve the question of how the sun generates its energy. While attending the annual meeting of the Washington Conference of Theoretical Physics, the participants worked out the possible path of reactions. One of the members of this group, was a Jewish German immigrant named Hans Bethe, who was a professor at Cornell University in New York.
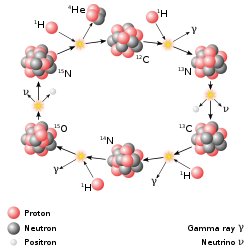
Upon returning from the conference, Hans Bethe and Charles Critchfield began their study of larger elements, and their possible generation within the sun, and larger stars. They discovered something remarkable when fused atoms within a sun’s core gained 6 or more protons: They could act like a Catalytic cycle to enhance the production of Helium‑4 from Hydrogen. A catalyst is a substance that does not get used up in a reaction and will continue to act repeatedly over and over again in a reaction. Bethe and Critchfield discovered that in the presence of atoms with 6, 7 and 8 protons, these atoms can facilitate the fusion of Hydrogen into Helium at a faster rate, working as a catalyst. This process is called the CNO-Cycle, since it requires larger atoms, the elements Carbon-Nitrogen-Oxygen to be present within a sun’s core. Our own Sun is a rather smaller Star, and as such contains a smaller amount of energy generated through the CNO-Cycle. It is estimated that only about 1.7% of the Sun’s energy is generated by the CNO-Cycle, however, in larger Stars the CNO-Cycle is an important process in the generation of energy, especially in stars of higher temperatures.
The discovery of the CNO-Cycle in the Sun and other Stars by Bethe, was marred by the rise of Adolf Hitler in Germany in the late 1930s. Hans Bethe, still a citizen of Germany, but of Jewish heritage, worked during this time to get his mother and family out of Germany. In fact, the paper describing the CNO-Cycle won a cash prize from the journal, which helped fund his mother’s emigration to the United States. Hans Bethe’s talent for understanding the physics of how nuclear fusion and fission worked was recognized by the United States military, who appointed him to lead the theoretical division for the top-secret Los Alamos Laboratories in the design and construction of the first nuclear weapons during World War II.
Even with the design and implementation of fission nuclear bombs in the 1940s, scientists were racing to figure out how larger atoms could form naturally within the heart of stars by the fusion of atoms.
Supernovas
[edit | edit source]
All this was spinning in Fred Hoyle’s mind as he lay at the base of the cliff. He knew all this. He had felt left out of nuclear research having served during World War II only in the capacity of a specialist in radar research. It was only after the war that he became passionately interested in the Sun’s nuclear fusion. How larger and larger atoms could form inside larger stars. Inspired by the research conducted in the United States during the war, Fred Hoyle developed the concept of nucleosynthesis in stars to explain the existence of elements larger than Helium‑4. Our own Sun generates its energy mostly by the Proton-Proton chain reaction, in other words, burning Hydrogen to form Helium‑4. With the occurrence of Carbon-Nitrogen-Oxygen, this process could be accelerated, but, Fred Hoyle pondered how elements larger than Helium‑4 could exist and be formed through the same process. He called this secondary process Helium-burning, a process of fusion of atoms together to produce even larger atoms, the numerous elements named on a periodic table of elements. Fred Hoyle, William Fowler and the wife and husband team of Margaret and Geoffrey Burbidge drafted a famous paper in 1957, which demonstrated that larger atoms could in fact be generated in very large stars, and that the natural abundances of those elements within surrounding planets led to greater knowledge of the steps taken to produce them in a star’s lifetime. These steps lead upward to the production of atoms with 26 protons, through a process of fusion of smaller atoms to make bigger atoms, but atoms with more than 26 protons required a special case: They formed nearly instantaneously in a gigantic explosion called a Supernova.
Thus, it was theorized that the basic distribution of elements within our solar system was formed through a stepwise process of fusion in a gigantic star that eventually went critical and exploded in a supernova event, injecting atoms of various sizes across a Nebula, a cloud of gas and dust blasted into outer space. This gas and dust, the Nebula, formed slowly over thousands of years into a protostellar-protoplanetary disk, that eventually led to the formation of our Solar System, and every atom within it. Carl Sagan often quoted this strange fact with the adage, “We are all made of stardust!”
Will the Sun Die?
[edit | edit source]
The fact that our Solar System formed from an exploding giant star, raises the question of what will happen to our Sun over time? The fuel of the sun is atoms of Hydrogen, in other words single Protons which over time are converted to Neutrons, or more specifically Helium‑4 atoms that contain 2 protons and 2 neutrons. Eventually, there will remain no more Hydrogen within the core of the Sun, as this fuel will have been replaced by Helium‑4. At this point, the Sun will contract, and compress inward and become more and more dense. At some point the increasing gravity will cause the Helium‑4 to fuse into larger atoms, and the Sun will begin a process of burning Helium‑4 as a fuel source, which will result in an expansion of the sun outward, well beyond its current size, forming a Red Giant. During this stage in its evolution, the Sun will engulf Mercury, Venus, and even Earth, despite burning at a cooler temperature. The Earth is ultimately doomed, but so is the Sun.
Eventually the Helium‑4 will be exhausted, and the Sun will contract for its final time, reducing its energy output drastically, until it forms a faint Planetary Nebulae, composed of larger atoms of burnt out embers of carbon, nitrogen, oxygen, and the remaining atoms of this former furnace of energy, until it is crushed to about the size of Earth. Scientists estimate that this process will take 6 billion years to play out, and that Earth, with an age today of 4.6 billion years, has about that many years remaining until our planet is destroyed during the Red Giant stage of our Sun’s coming future.
The Big Bang
[edit | edit source]When Fred Hoyle tried to rise himself up from his splayed position below the cliff, he winced in pain, bringing back the memory of his most noteworthy quote, a phase he mentioned during a radio interview in 1949. A phase that suggested that not only that the Solar System had its beginning with a violent explosion, but that a much older explosion birthed the entire universe. Hoyle rejected an origin of the universe vehemently, favoring an idea that the universe has always existed, and that it lacked any beginning. During the 1949 radio interview, Hoyle explained his steady state hypothesis, by contrasting his ideas to the notion of a “Big Bang”, an explosive birth of the universe. A new idea that was proving interesting to other scientists but Hoyle rejected it. During the 1960s, Hoyle begin rejecting any idea that seemed to contradict his own, and became noteworthy for his contrarian scientific views. In 1962, a young student named Steven Hawking applied to study with Fred Hoyle at Cambridge, but was picked up by another professor to serve as his advisor. This was a good thing, as Hoyle became entrenched in his idea that the universe had no beginning, so much so, that in 1972 after a heated argument with his colleagues over hiring practices, Hoyle quit his teaching position at the university and retired to the countryside. That same year he had received a knighthood, and he struck out on his own.
However, the memory of that period likely brought a painful sensation to his heart. Outside of the academic halls, Fred Hoyle became a burr toward the established scientists of the day, drafting more and more controversial and strange ideas and finding some success in publishing science fiction novels with his son. In 1983, he was excluded by the Nobel prize committee, which awarded the prize to his co-author William Fowler and Subrahmanyan Chandrasekhar for their work on stellar nucleosynthesis. Snubbed, Fred Hoyle fell into obscurity. However, the concept of a “Big Bang” would come to define the future exploration of theoretical physics, and make Steven Hawking a household name.
Although Fred Hoyle was rescued from his fall from the cliff and transported to the hospital, he never recovered from his fall from science. A fall that could have been averted if he had observed the growing number of breakthroughs regarding the nature of light, and startling discoveries that proved the universe did indeed have a beginning.
| Previous | Current | Next |
|---|---|---|
