Planet Earth/5a. H2O: A Miraculous Gas, Liquid, and Solid
Water on Earth
[edit | edit source]
Water (H2O) is the most abundant substance on Earth’s surface and one of the most abundant molecules in the universe. Liquid water covers 71% of the Earth’s surface leading to the striking blue color of Earth when viewed from space. As the only dark blue planet in the solar system, Earth is unique in its idyllic location from the sun to facilitate all three phases of H2O, as liquid water in the oceans, water vapor in the clouds, and ice in Earth’s glaciers and snow. One of the most amazing features of planet Earth is that throughout its long history all three phases of H2O were present.

On the ice-covered moon of Jupiter, Europa, water remains locked beneath a frozen sea of ice in the extreme permeant cold temperatures well below -150 degrees Celsius, while Mars with an average temperature of -60 degrees Celsius, water exists only as frozen ice, principally at its northern pole.

Evidence for the early presence of liquid water on Mars has been discovered through the Curiosity Mars rover, the Mars Reconnaissance Orbiter and other missions to the planet, indicating that Mars may have been warmer in its early history several billion years ago.

Venus, a planet slightly closer to the sun than Earth, has an average temperature of 462 degrees Celsius, much hotter than the boiling point of water, and all the water on Venus is found as gas in the form of water vapor in the thick hot atmosphere above its surface. Even Earth’s own moon lacks significant liquid and gas phases of water, despite a daily range from -173 degrees Celsius at night to 127 degrees Celsius during the day, the extreme daily dehydration and rehydration of the trace water in the rocks and dust on the moon have led to nearly permanent frozen ice to accumulate near the cold poles and in icy shadows from the sun’s daily heat.

Earth’s dynamic liquid water of the oceans, water vapor in atmosphere, and snow and ice are truly unique and special in the solar system. This is due to the fine balance of planetary temperatures ranging across the melting and boiling points of water, but not being too extremely cold or hot within that range.
Water, the Molecule
[edit | edit source]
With the chemical formula of H2O, each molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. At 1 atmosphere of pressure (sea level), water cooled to 0 degrees Celsius (32 degrees Fahrenheit) will freeze into ice, and when heated to 100 degrees Celsius (212 degrees Fahrenheit) water boils into steam or water vapor. The “triple point” is a point on a phase diagram illustrating the state of a substance at various temperatures and pressures where all three states (gas, liquid, solid) can coexist. The triple point of water occurs at 0.0075 °C (32.0135 °F) and 611.657 pascals or 0.006 atmospheric pressure. This point is similar to the cold temperatures that freeze water at normal atmospheric pressures at sea level, but a triple point can occur naturally in the very low atmospheric pressures 36 kilometers above Earth’s surface, resulting in ice, water and water vapor to coexist in the stratosphere, high above Earth.

Water, ice, and water vapor has a broad absorption band that includes long-wave lengths of electromagnetic radiation beyond the visible spectrum, including infra-red light and microwave lengths of radiation. In the visual spectrum molecules of water and ice weakly absorb wave lengths down to 750 nanometers in length, which block some visible red light-waves, resulting in water and ice having a bluish color. Water’s broad band of absorption of infra-red electromagnetic radiation results in a high heat capacity of water.

In fact, the specific heat capacity of water is one of the highest among common molecules. Water also exhibits high heat of vaporization, which resists water from boiling before reaching the boiling temperature. Frozen water has a high specific enthalpy of fusion or latent heat, which means that it takes a lot of energy to melt ice and raise the temperature of water compared to other types of molecules. As a consequence of these three unusual thermal properties, water and its distribution on Earth has a profound effect on Earth’s climate, because it can store enormous amounts of heat and can resist warming as frozen ice. The specific heat capacity of ice at −10 °C is 2.03 J/(g·K) and the heat capacity of steam at 100 °C is 2.08 J/(g·K). These unusual properties (high heat capacity, high heat of vaporization, and high specific enthalpy of fusion) are a result of the strong hydrogen bonds formed between individual molecules.
Hydrogen bonding in Water
[edit | edit source]
In liquid water, hydrogen atoms within water molecules are attracted to oxygen atoms of neighboring molecules because of a slight polarization of the water molecule. The oxygen atom contains 8 protons (+8 charge) and will attract orbiting electrons slightly more than hydrogen atoms with 1 proton (+1 charge). This means that the oxygen atom within a water molecule carries a partial negative charge, due to the negative charged electrons attracted to the oxygen nucleus, while hydrogen atoms will carry a slight partially positive charge. This polarization results in molecules of water oriented so that hydrogen atoms are attracted to neighboring molecules. This attraction is weak, and easily broken. The lifespan of an individual hydrogen bond in water is very very short, with new hydrogen bonds consistently being formed and broken in a glass of water. This is very different then the strong covalent bonds that hold the single atom of oxygen and two atoms of hydrogen together. Those covalent bonds require considerable energy to break, and are strongly bonded.

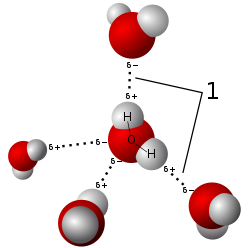
Despite its weakness, hydrogen bonding is important because it leads to the unique chemical properties found in water. Liquid water has high cohesion. Cohesion is the attraction molecules have with each other. Hydrogen bonding makes the molecules of liquid water attract each other, making them stick together, and giving water the appearance of a “skin” when forming water droplets. When liquid water molecules stick together they form spherical drops due to the weak hydrogen bonding holding these molecules together, this is called cohesion. This cohesion results in strong surface tension, which is the property of the surface of a liquid that resists an external force. For example, if you are careful, you can float a paper clip on the surface of liquid water, due to this high surface tension. The high surface tension of water allows insects like water skippers of the family Gerridae to walk on this surface without sinking into the liquid water. Water molecules also exhibit slight adhesion with other molecules, particularly those with oxygen (like silica glass SiO2) and hydrogen atoms (like hydrocarbons found in plastics (C2H4)x polymers). This adhesion causes a meniscus to form near the edges of a glass.

A meniscus is a concave depression, caused by water molecules clinging to the surface of a solid, such as a test tube, plastic flask or wine glass. This is caused by the adhesive of the weak hydrogen bonds between the water molecules and the neighboring atoms of oxygen or hydrogen they come in contact with. This adhesion can result in capillary action, allowing water to be pulled up narrow skinny tubes, which are found in nature in roots and stems of vascular plants that require water, and even in blood vessels in living animals.
Water, the Universal Solvent
[edit | edit source]The unique polarization of water molecules, with slight positive and negative charges on each side, facilitates liquid water molecules to break ionic bonds of solids placed within liquid water. This allows water to dissolve solids, such as salt by breaking the weak ionic bonds between the atoms in the solid. A liquid capable of dissolving solid substances formed by weak ionic bonds is referred to as a solvent. Water is highly regarded as one of the most important solvents on Earth, and can dissolve more solid substances than any other liquid.

Composed of cations of Na+ and anions of Cl− attracted to each other by their opposite electric charge, table salt when placed in liquid water breaks apart and dissolves. The Na+ will be attracted to the oxygen-side of water molecules that carry a slight negative charge, while Cl− will be attracted to the hydrogen-side of water molecules, breaking the ionic bond between Na+ and Cl−, that forms solid salt crystals. These ions will exist within the salty liquid, having been dissolved within the liquid water. If more salt is added to the liquid water, the liquid will become supersaturated. A supersaturated liquid is a solution that contains more of the dissolved material than can be dissolved by the solvent, and will start to precipitate from the water. The amount of solid that can be dissolved in a liquid is dependent on temperature. This is often used in making candy, where sugar is added to boiling water, as the water cools the amount of sugar that can be dissolved decreases, and sugar crystals will form. One of the most interesting chemical properties occurs when molecules contain hydrogen H+ or hydroxide OH− ions are added to water.
pH: Acids and Bases
[edit | edit source]

Working in the 1890s Johan Kjeldahl, a Danish chemist at the Carlsberg Brewery was given the task of finding out how much protein was in grain to be used to make malt for beer production. The less protein in the grain used at the brewery, the more beer could be made, since protein is not needed in the process of alcohol fermentation. Kjeldahl was successful and developed a method to measure the amount of nitrogen in the grain, which is found in protein, but not in sugars. Thus, the amount of nitrogen found in the grain, the more protein the grain had. One day, in July of 1900 Johan Kjeldahl died suddenly at the age of 50. His vacancy at the brewery attracted the attention of a young Danish chemist named Søren Peter Lauritz Sørensen. Sørensen grew up wanting to become a doctor, but became interested in inorganic chemistry and geology. During college he worked the summers on geological surveys of Denmark, but his real passion was chemistry. He had hope to become a teacher, but the position at Carlsberg Brewery paid much more, and he secured the job at the brewery. He took up a new task in understanding how proteins and other complex organic molecules can be broken down. Proteins can be broken down by heating them up to hot temperatures, and brewers boiled the grains to make malt, but it was also known that acids could break down proteins as well. Working at the brewery Sørensen undertook a careful study of how acids work.
Acids are formed when molecules of a substance containing ionic bonds that include hydrogen is added to water, for example HCl (hydrochloric acid). The hydrogen H+ cations will separate from the Cl− anions resulting in dissolution, like in salt (NaCl). However, the hydrogen H+ cations are highly reactive once dissolved and will react with complex organic proteins breaking them apart. What makes a liquid an acid is how many hydrogen H+ cations are dissolved within the liquid. One way to neutralize these excess hydrogen H+ cations is to introduce a substance containing OH− anions, like Ca(OH)2 (calcium hydroxide). These OH− anions will react with the H+ cations and form H2O. Liquids that contain an excess of OH− anions are called basic, while liquids that contain an excess of H+ cations are called acidic. Pure water (H2O) will contain no excess H+ cations, nor excess OH− anions, and will be neutral.
In his laboratory at the brewery, Sørensen needed to develop a method to classify various liquids into a scale of how acidic or basic they were in his experiments. In 1909 he developed a logarithmic scale now widely used in chemistry, geology, and biology. Later modified in 1924 into the pH scale.
Sørensen knew that there would always be a tiny amount of H+ cations, even in liquids with abundant OH− anions. The amount of H+ cations would be exponentially smaller and smaller the more OH− anions are added. In neutral water Sørensen found that active H+ cations was only 0.000000003540133 per mole, having to write out such small numbers with so many zeros was not particularly useful, so instead Sørensen developed a simple method using an inverse logarithmic scale, which means the larger the value of H+ cations in a liquid, the smaller the number on the scale would be.
A liquid with 0.5 H+ cations per mole would have a very low pH of 0.3, while a liquid with a tiny amount, say 0.0000000001 H+ cations per mole would have a high pH of 10. Liquids with pH below 7 are acidic, while liquids with pH above 7 are basic. Bleaches and other household cleaners on this scale have around 13.5 pH, while vinegar is around 3 pH. Extremely low and high pH can easily break down proteins, with an excess of either H+ cations or OH− anions. These liquids on the high and low end of the scale are highly corrosive and dangerous, including liquid drain cleaner (very high pH) and battery acid (very low pH). Understanding pH is really important in understanding Earth’s water, because water is a powerful solvent, and will break ionic bonds resulting in mixtures of water with varying amounts of H+ cations or OH− anions. This will be particularly important in understanding the chemistry of rain, groundwater, rivers, lakes and oceans on Earth.
| Previous | Current | Next |
|---|---|---|
|
b. Properties of Earth’s Water (Density, Salinity, Oxygen, and Carbonic Acid). |

