Principles of Biochemistry/Enzymes
Enzymes are organic catalysts that are important to all living things due to the continuous-controlled chemical activities in cells. Enzymes regulate metabolism by altering the rate of chemical reactions. Activation energy is decreased in order to alter chemical reaction rates. In addition, enzymes are able to transform one form of energy to another. Due to the fact that enzymes are highly selective, they may only catalyze one or a specific class of reactions. The place where an enzyme binds onto the substrate is called an active site. A substrate is the molecule that enzyme acts upon.
There are two theories that describe the binding of enzymes: 1) Lock and Key Theory and 2) Induced Fit Theory.
1) Lock and Key Theory: The shape of the enzyme's active site is complementary to that of its substrate
2) Induced Fit Theory:
The active site has a flexibility of shape, thus when an appropriate substrate comes in contact with the enzyme's active site, the shape of the active site would change to fit with the substrate.
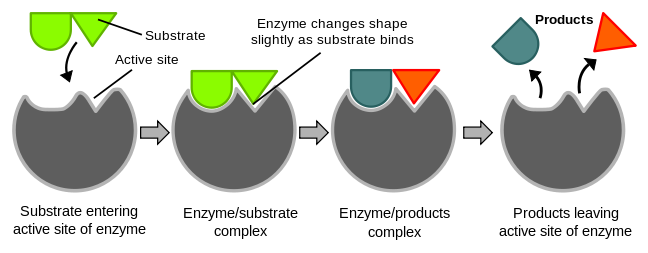
Figure 1 demonstrates the induced fit theory. Reference: en.Wikipedia, author: TimVickers
Temperature
Because most enzyme reactions are reversible, an enzyme can synthesize and decompose molecules. Enzymes reaction rate is dependable on several factors: pH, temperature, and concentration of both the enzyme and substrate. Generally, the rate of enzyme reaction would increase as temperature increase; however, if the optimal temperature—usually around 40°C-- is reached the enzyme would denatured and loss its ability to react with the substrate.
pH
Despite the fact that every enzymes has their own optimal pH value, most enzyme reactions have higher reaction rates between pH value 6 - 9. The maximal activity rate of enzyme reaction in human body is around pH 7.2, with the exception of pepsin, which has a high reaction rate under acidic condition ( usually around pH 2), and pancreatic enzyme, which has a high reaction rate under alkaline condition ( usually around pH 9)
Concentrations
Furthermore, concentrations of substrates and enzymes affect the reaction rates as well. The lower the concentration of enzymes and substrates are the slower the reactions, because less active sites on enzymes are occupied. On the other hand, higher concentration of enzymes and substrate lead to the increase of reaction rate as more active sites are occupied. However, the reaction rate will no longer increase once the reaction velocity of the enzyme reaction reached a certain level.

Figure 2 shows the relationship between concentration and reaction rate. Reference: en.Wikipedia, author: fullofstars
Enzyme Inhibitors There are two categories of enzyme inhibitors: 1) Reversible inhibitors and 2) irreversible inhibitors. Reversible inhibitors are kinetically distinguishable, and is characterized by rapid dissociation of enzyme-inhibitor complex.
There are three sub-categories in reversible inhibitors: 1) Competitive 2) Uncompetitive and 3) Non-competitive Competitive inhibition
Although the active site of an enzyme is highly specific, it is possible that molecules with similar shape to the substate to bind to the active site. Active sites bounded to a molecule will interfere with enzyme reaction. Therefore, the possibility of substrates bind to active sites are lower, thus, known as competitive inhibition. However, competitive inhibition happens only when similar about of molecule and substrate are present. In competitive inhibitors, enzymes can only bind to either the substrate or inhibitor but not both. In addition, during competitive inhibition, concentration of substrate increases as the maximum velocity remains constant.

Figure 3 demonstrates how competitive inhibition of enzymes work. Reference: en.Wikipedia, Authored by Jerry Crimson Mann, modified by TimVickers, vectorized by Fvasconcellos
Uncompetitive inhibition In an uncompetitive inhibition, inhibitor only binds to enzyme-substrate complex. In addition, in an uncompetitive inhibition, both concentration of substrate and maximum velocity decrease.
Noncompetitive inhibition
A noncompetitive inhibition reaction is nonreversible due to the act that strong covalent bonding, that cannot be displaced by the addition of excess substrate, between a molecule and a enzyme. That molecule is also called a noncompetitive inhibitor. In non-competitive inhibition, inhibitor and substrate can bind to enzyme simultaneously at different binding sites. Furthermore, it can bind free enzyme or enzyme substrate complex. Also, during a noncompetitive inhibition, concentration of substrate will increase while maximum velocity will decrease.
There are three sub-categories of irreversible inhibitors: 1) group specific, 2) reactive substrate analogs, 3) mechanism-based inhibitors. Group Specific inhibitors only react with specific side chains of amino acid or with certain chemical groups.
Reactive substrate analogs, also known as affinity label. In reactive substrate analogs, molecules that are structurally similar to the substrate for an enzyme and that covalently bind to active-site residue.
Mechanism-based inhibitors is also known as suicide inhibitors. There are three steps in the mechanism, the first one is oxidation which is then followed by alkylation and lastly by protonation.
Cofactors
Cofactors are needed for some catalytic reactions. Cofactors can either be inorganic elements (metals) or complex organic molecules, in which many of them are derived from vitamins. A holoenzyme is a catalytically activated enzyme; while an apoenzyme is an enzyme without cofactor.
Gibbs Free Energy (ΔG)
Enzymes decrease activation energy. Enzymes accelerates reactions by lowering Gibbs free energy at transition state, which is also known as the free energy of activation. Gibbs free energy also provides information about spontaneity of a reaction.
Reaction is spontaneous if : ΔG<0
Reaction is not spontaneous if : ΔG > 0
Reaction is at equilibrium if : ΔG=0
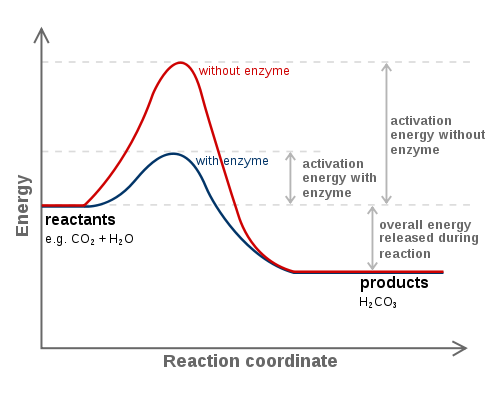 This figure shows the catalytic reaction of enzyme.
Reference: Wikipedia, author: Fvasconcellos (talk · contribs).
This figure shows the catalytic reaction of enzyme.
Reference: Wikipedia, author: Fvasconcellos (talk · contribs).
To determine the total Gibbs free energy of a reaction, take the summation of Gibbs free energy of formation of products and subtract the sum of Gibbs free energy of formation of reactants from it. Another important point regarding ΔG is that ΔG does not explain the reaction rate of a reaction.
Active Site
An active site of an enzyme is where substrate bind to and is also where chemical reaction take place. An active site is a small part on a protein and is cleft, or crevice. It also has a unique microenvironment and substrates are bind by several weak interactions. furthermore, specificity of the enzyme depends on the precise arrangement of atoms.
Biochemical Reactions
There are two main enzymatic reactions: 1) Sequential Reactions and 2) Double Displacement Reaction
1) Sequential Reaction: In sequential reactions, all substrates are bind to the enzyme before any product is released. In an ordered sequential reaction, substrates bind to enzyme in a defined sequence; while in a random sequential reaction, substrates bind to enzyme in an undefined sequence.
2) Double Displacement Reaction: In double displacement reactions, one or more products are released before all substrates bind to the enzyme.
References
[edit | edit source]- ↑ Kaplan PCAT 2010-2011 Edition
