Proteomics/Post-translational Modification/Amino Group Modification
This Section:
Amino Group Modification
[edit | edit source]
Amino group modification involves the addition of a functional group at the N terminus of the amino acid.
A protein after its translation undergoes a chemical modification called Post Translational Modification. These modifications may alter the functions of the protein when attached to a biochemical functional group like acetate by changing the chemical nature of the amino acid or by structural changes like folding, conformation distribution, stability, activity etc.
Types of Amino Group Modifications
[edit | edit source]Acetylation
[edit | edit source]Acetyaltion is an acylation (introduction of an acyl group to an organic compound) process which involves the substitution of an organic group of acetic acid for an active hydrogen atom at the N-terminus.
The most widespread modification in the eukaryotes is the Acetylation of the N-terminal α-amine group of proteins. About 50% of the proteins of yeast and approximately 90% of the proteins in humans are modified by this mechanism. The pattern of the modification is conserved all through the evolution. Even though it is such a common modification, not much information is available for about the biological function of N-α-terminal acetylation. N-α-acetyltransferase (NATs) is the enzyme responsible for the Acetylation. NATs belong to a family of the GNAT, which lies under the superfamily of the acetyltransferases. [1]
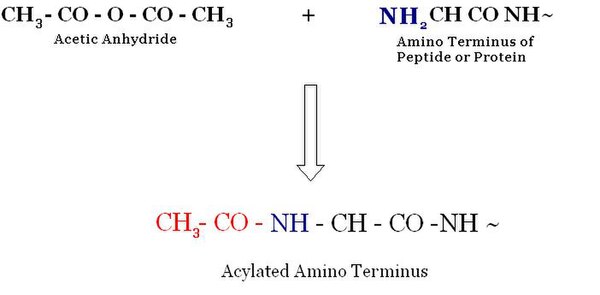
The acetylation and deacetylation takes place on lysine residues in the N-terminal tail in histone acetylation and deacetylation. These reactions take place in the presence of the enzymes histone acetyltransferase (HAT) or histone deacetylase (HDAC).
Pyroglutamate Formation
[edit | edit source]
Pyroglutamate is formed through the cyclization (ring formation in the chemical compound) of glutamine. It is commonly observed in antibodies that contain glutamate or glutamine residues at their N-termini. The amino group and the glutamate or glutamine condenses to form a five-member ring called Pyroglutamate. This residue makes the protein more resistant to aminopeptidases and has many functional roles.[2]
It maintains structural integrity at the N-terminal α-helix and provides proper environment for the ionization of Histidine residues for catalysis and cytotoxicity against HeLa cells.
Myristoylation
[edit | edit source]N-myristoylation is also an acylation process found to be specific to N-terminal amino acid glycine in proteins where a myristoyl group (derived from myristic acid) is covalently attached via an amide bond to the alpha-amino group of N-terminal Glycine. Myristoylation plays vital role in the secondary cellular signaling, in the infectivity of retroviruses and oncogenesis in Eukaryotes. It also influences the physiological functions of calcium binding proteins.[3] The cytosolic enzyme N-myristoyltransferase (NMT) catalyzes Myristoylation.
Methylation
[edit | edit source]Methylationof the protein is the most common form of post-translational modifications observed. Similar to other post-translational modifications, protein methylation is involved in regulating protein-protein interactions resulting in a plethora of effects during key cellular events, including regulation of transcription [4] [5] [6]stress response, ageing and protein repair[7] T-cell activation [8], nuclear transport [9], neuronal differentiation [10],[11] ion channel function, and cytokine signaling. A protein is considered methylated when a methyl group is added at one or more nucleophilic side chains. Methylation on side chain nitrogens is considered largely irreversible while methylation of the carboxyl groups is potentially reversible. Protein residues methylated on nitrogen include the e-amine of lysine, the imidazole ring of histidine, the guanidino moiety of arginine, and the side chain amide nitrogens of glutamine and asparagine.

Methylation in the proteins negates the negative charge on it and increase the hydrophobicity of the protein. Methylation on carboxylate side chains cover up a negative charge and add hydrophobicity. N-Methylation of lysines does not alter the cationic charge but does increase hydrophobicity. In particular, dimethylation and trimethylation of lysine side chains in proteins increase both hydrophobicity and steric bulk and can affect protein–protein interactions if they are in an interacting surface.
Carbamylation
[edit | edit source]Carbamylation occurs when isocyanic acid (HCNO) reacts with the amino terminus residues, like lysine, of the protein. It is one of the common artifactual protein modifications recognized to Isoeletric focusing. The risk factor is urea (chaotrope) which exists in solution and is in equilibrium with ammonium cyanate. Isocyanic acid is the cyanate form that reacts with amino groups of proteins. For carbamylation to occur the amino acid groups of proteins like lysine, arginine side chains should be deprotonated which usually occurs at alkaline pH. Carbamylation occurs when proteins are left at room temperature in a solution of urea and where isocyanic acid can freely react with the protein.
Carbamylation by isocyanic acid is negative for next steps of protein characterization because isocyanic acid reacts with the amino terminus of proteins blocking the peptide or protein to N-terminal sequencing. Isocyanic acid attacks the side chains of lysine and arginine residues making a protein unsuitable for many enzymatic digests. Even if carbamylation does not prevent the enzymatic digest it will often confuse the results from mass spectroscopy experiments with peptides that have unexpected retention times and masses. Carbamylation of proteins in vivo were observed in several diseased states.[12]
Formylation
[edit | edit source]Formylation is one of the posttranslational modifications of the protein, in which a protein is modified by the attachment of formyl group. The most commonly studied mechanisms is the N6-formylation of lysine which is associated with the histone and other nuclear proteins. The post translational modification of histone and other chromatin proteins play a role in the physiology of gene expression. "The N6-formyl-lysine residue appears to represent an endogenous histone secondary modification, one that bears chemical similarity to lysine N6-acetylation recognized as an important determinant of gene expression in mammalian cells." From the study it was concluded that N6-formyl modification of lysine is interfering with the signaling functions of acetylation and methylation, which play a role in the physiology of oxidative and nitrosative stress.[13]
References
[edit | edit source]- ↑ Wikipedia : Acetylation [1]
- ↑ Post-translational modification of bovine pro-opiomelanocortin. Tyrosine sulfation and pyroglutamate formation, a mass spectrometric study. A Bateman, S Solomon and HP Bennett.J. Biol. Chem., Vol. 265, Issue 36, 22130-22136, 12, 1990[2]
- ↑ N-terminal Myristoylation Regulates Calcium-induced Conformational Changes in Neuronal Calcium Sensor-1. Andreas Jeromin, Dasari Muralidhar, Malavika Nair Parameswaran, John Roder, Thomas Fairwell, Suzanne Scarlata, Louisa Dowal, Sourajit M. Mustafi, Kandala V. R. Chary, and Yogendra Sharma J. Biol. Chem., Vol. 279, Issue 26, 27158-27167, June 25, 2004[3]
- ↑ Clarke S: Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair.Ageing Research Reviews 2003, 2(3):263-285.
- ↑ Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O: CD28 costimulatory signal induces protein arginine methylation in T cells. Journal of Experimental Medicine 2005, 202(3):371-377.
- ↑ Smith WA, Schurter BT, Wong-Staal F, David M: Arginine methylation of RNA helicase a determines its subcellular localization.Journal of Biological Chemistry 2004, 279(22):22795-22798.
- ↑ Kujubu DA, Stimmel JB, Law RE, Herschman HR, Clarke S: Early responses of PC-12 cells to NGF and EGF: effect of K252a and 5'-methylthioadenosine on gene expression and membrane protein methylation. Journal of Neuroscience Research 1993, 36(1):58-65.
- ↑ Cimato TR, Ettinger MJ, Zhou X, Aletta JM: Nerve growth factor-specific regulation of protein methylation during neuronal differentiation of PC12 cells. Journal of Cell Biology 1997, 138(5):1089-1103
- ↑ Vemuri R, Philipson KD: Protein methylation inhibits Na+-Ca2+ exchange activity in cardiac sarcolemmal vesicles.Biochimica et Biophysica Acta 1988, 939(3):503-508.
- ↑ Chen YF, Zhang AY, Zou AP, Campbell WB, Li PL: Protein methylation activates reconstituted ryanodine receptor-ca release channels from coronary artery myocytes.Journal of Vascular Research 2004, 41(3):229-240.
- ↑ Mowen KA, Schurter BT, Fathman JW, David M, Glimcher LH: Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes.Molecular Cell 2004, 15(4):559-571.
- ↑ A turning point in proteome analysis: sample prefractionation via multicompartment electrolyzers with isoelectric membranes.Herbert B, Righetti PG.[4]
- ↑ Nepsilon-formylation of lysine is a widespread post-translational modification of nuclear proteins occurring at residues involved in regulation of chromatin function (PMID: 18056081). Wisniewski JR, Zougman A, Mann M. [5]



