Proteomics/Protein Separations- Electrophoresis/Two Dimensional Polyacrylamide Gel Electrophoresis(2D-PAGE)
Theory
[edit | edit source]Two dimensional polyacrylamide gel electrophoresis (2D-PAGE) is a form of gel electrophoresis in which proteins are separated and identified in two dimensions oriented at right angles to each other.
In this technique, proteins are separated by two different physical properties. The first dimension, isoelectric focusing, separates proteins on the basis of their net charge. The second dimension, SDS-PAGE, further separates the proteins by their mass. Small changes in charge and mass can easily be detected by this method, because it is rare that two different proteins will resolve to the same place in both dimensions.[1]
For further details on gels, staining, and electrophoretic analysis, see the "Gel Electrophoresis" section of this chapter. For further details on the individual dimensions of two dimensional electrophoresis, isoelectric focusing and SDS-PAGE, see the "One Dimensional Electrophoresis" section of this chapter.
Process
[edit | edit source]The proteins in a focused IPG strip are uncharged (their net charge is 0) because they are at their isoelectric point, so they will not move into the SDS-PAGE gel. To prepare the proteins for movement into the SDS-PAGE gel, the equilibrated strip is treated with detergent SDS. The treated IPG strip is placed on top of the SDS-PAGE gel, submerged in a buffer, and sealed in place with agarose gel. When an electric current is applied across the layered gels, the negatively charged proteins move out of the IPG strip and migrate through the SDS-PAGE gel. Each protein moves differently through the gel matrix depending on its size. The separation of proteins is distributed roughly according to size (molecular weight), like other electrophoresis methods.[2]
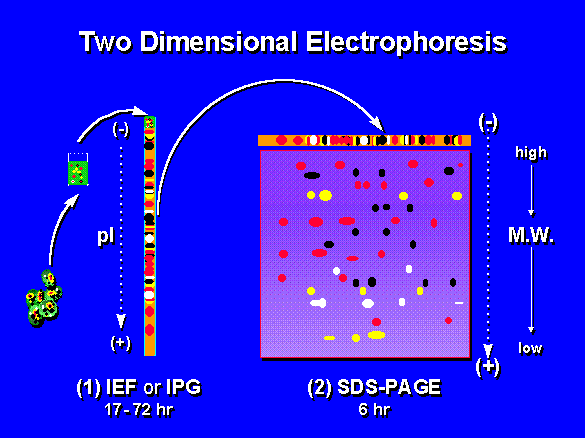
Visualization and Analysis
[edit | edit source]The resolution of 2D-PAGE gels is somewhat different than that of one-dimensional gel electrophoresis. Proteins are rarely resolved as discrete bands; rather, they are resolved as spots cascading down the gel. These gels can be stained in much the same way as other electrophoresis techniques, and adding another dimension of separation does not in any way hinder visualization.
Some analysis of 2D-PAGE gels has a direct complement in one-dimensional electrophoresis; the determination of molecular weight is done the same way in 2D-PAGE as in the simpler method. Analysis of 2D-PAGE gels can become more complicated, however, because it is not as easy to discern the identity of proteins with the naked eye. For careful analysis, image analysis software, such as Delta2D, can be useful. Delta2D can quantify protein spots, match images, compare corresponding spots and intensities of related gels, prepare gel data reports, remove background patterns, and integrate image information to databases.[3] There are also many other software packages available.
Advantages and Disadvantages
[edit | edit source]Using 2D-PAGE, hundreds to thousands of polypeptides can be analyzed in a single run. The proteins can be separated in pure form from the resultant spots. The spots can be quantified and further analyzed by mass spectrometry, depending on their resolution. Polypeptides can also be probed with antibodies and tested for post-translational modifications. 2D-PAGE is also used to study differential expression of proteins between cell types.
The disadvantages of this technique include a large amount of sample handling, limited reproducibility, and a smaller dynamic range than some other separation methods. It is also not automated for high throughput analysis. Certain proteins are difficult for 2D-PAGE to separate, including those that are in low abundance, acidic, basic, hydrophobic, very large, or very small. Through advancements in this technology, scientists are, however, able to separate low abundance proteins with moderate efficiency.


