Radiation Oncology/Eye/Ocular melanoma
Appearance
|
Ocular Melanoma
- Subpage: Randomized evidence
Epidemiology
[edit | edit source]- US incidence 1,350 cases/year (0.7 per 100,000)
- Most common primary intraocular malignancy (75%) [#2 retinoblastoma (13%)]
- Median age is 55 yr
- More common in caucasians (8 to 1 caucasian to AA; 3 to 1 caucasian to asian)
- Arise from uveal melanocytes which originate from neural crest cells
Risk Factors
[edit | edit source]- Intensive UV light exposure - RR 4.0-7.0
- Increasing age
- Ocular and oculodermal melanocytosis (ie. nevus of Ota) - malignant transformation in 4.6%
- Pigmented iris nevus
- Familial - rarely occurs in dysplastic nevus syndrome
- Family history of cutaneous or ocular melanoma
- Light iris color
- Light skin pigmentation & tendency to sunburn - RR 2.0
Clinical Presentation
[edit | edit source]
- Approximately 1/3 patients asymptomatic, as incidental finding
- Visual symptoms are variable, depending on location
- Diagnosis is typically not based on histopathology due to high risk of obtaining tissue and the risk of tumor dissemination, combined with high diagnostic accuracy of ultrasound
- Clinical diagnostic accuracy rate in oncology centers is ~99%
- Unpredictable clinical course, with late (46 years) fulminant failures possible
- Metastatic spread on presentation is rare (~1%) and is typically to liver (60-90%), subcutaneous nodules, lung
- Work-up should include chest x-ray and liver LFTs and CT scan
Anatomy
[edit | edit source]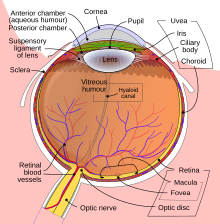
- Melanomas develop in the uveal tract:
- Vascular support layer located between the sclera and the retina
- Divided into iris, ciliary body, pars plana and choroid
- Melanoma location:
- 80% choroid
- 10-15% ciliary body
- <10% iris
- Size:
- Precise size should be obtained by ultrasound
- Size can be estimated in average optic disc diameters (1 dd = 1.5 mm)
- Height can be estimated in diopters (3 diopters = 1mm)
- Can bulge into the retina, giving rise to pathognomonic "collar button" deformity
Staging
[edit | edit source]COMS
[edit | edit source]| Stage | Apical Height | Basal Diameter |
|---|---|---|
| Small | <3 mm | 5 – 16 mm |
| Medium | 3 – 10 mm | 5 – 16 mm |
| Large | >10 mm | >16 mm |
| Diffuse | Flat growth, thickness <20% basal dimension | |
| Metastatic | Any N1 or M1 | |
AJCC 2002
[edit | edit source]| Stage | Apical Height | Basal Diameter |
|---|---|---|
| T1 | <=2.5 mm | <=10 mm |
| T1a | No extraocular extension | |
| T1b | Microscopic extraocular extension | |
| T1c | Macroscopic extraocular extension | |
| T2 | >2.5 – 10 mm | >10 - 16 mm |
| T2a | No extraocular extension | |
| T2b | Microscopic extraocular extension | |
| T2c | Macroscopic extraocular extension | |
| T3 | >10 mm | >16 mm |
| T4 | T3 with extraocular extension | |
| N | N0 or N1 | |
| M | M0 or M1 | |
| Stage | Description | |
|---|---|---|
| T1 | Limited to iris only | |
| T1a | <=3 clock hours in size | |
| T1b | >3 clock hours in size | |
| T1c | With melanomalytic glaucoma | |
| T2 | Extending into ciliary body and/or choroid | |
| T2a | With melanomalytic glaucoma | |
| T3 | Extending into sclera | |
| T3a | With melanomalytic glaucoma | |
| N | N0 or N1 | |
| M | M0 or M1 | |
Pathology
[edit | edit source]- Spindle Cell (30%):
- Type A - Best prognosis, 5-yr OS rate = 95%
- Type B - Intermediate prognosis, 5-yr OS rate = 85%
- Mixed Cell (65%):
- Contains spindle and epithelioid cells, 5-yr OS rate = 83%
- Epithelioid Cell (5%):
- Worse prognosis, 5-yr OS rate = 60%
Treatment Overview
[edit | edit source]- Survival after development of metastatic disease is poor (median 7 months), so main goal of treatment is cure while disease is confined to the eye
- Historically, enucleation was the standard of care; however, as many as 20% cases were clinically misdiagnosed
- Review of data published in 1978 suggested that enucleation may increase risk of metastasis, and subsequent death. Subsequent analyses challenged this observation, but search for alternative treatments was on
- Retrospective studies in 1980's suggested similar outcomes between radiation therapy (protons or cobalt plaque) and enucleation
- A randomized trial comparing Helium ions and I-125 plaque brachytherapy showed that 70 Gy delivered at the I-125 dose rate is insufficient for control
- The Collaborative Ocular Melanoma Study (COMS) is a set of clinical trials that was carried out in 1980's - 1990's to evaluate the role of radiation therapy in management of ocular melanoma:
- Small tumors (height 1 - 3 mm, diameter >5 mm) were prospectively observed
- Medium tumors (height 2.5 - 10.0mm and diameter <=16 mm) were randomized to I-125 plaque brachytherapy (85 Gy) compared with enucleation
- Large tumors (height >=10 mm or diameter >=16 mm) were randomized to pre-enucleation EBRT 20/5 vs. enucleation only
- The COMS "Small" study showed that with prospective follow-up, overall survival is comparable to general population
- The COMS "Medium" randomized trial showed that the overall survival and risk of death from metastatic disease were comparable between enucleation and plaque brachytherapy, establishing plaque brachytherapy as a reasonable primary treatment
- The COMS "Large" randomized trial revealed that pre-enucleation RT does not provide any additional benefit.
- While there have been efforts at non-enucleation treatment approaches for large lesions, none have sufficiently long follow up. Also, the benefit of greater useful vision preservation appears unlikely
- to be continued ...
Natural History
[edit | edit source]- COMS Declined Treatment; 2003 PMID 12834669 -- "Mortality after deferral of treatment or no treatment for choroidal melanoma." (Straatsma BR, Am J Ophthalmol. 2003 Jul;136(1):47-54.)
- Prospective. Patients eligible for COMS who deferred treatment. 77 patients deferred, 45 enrolled in this study, 42 medium melanoma. Median F/U 5.3 years
- Outcome: 52% had subsequent melanoma treatment. 5-year medium melanoma OS 70% compared with enrolled COMS patients 82% (NS). Risk of death 1.54X (0.93-2.56). Metastatic disease in 42% deaths
- Conclusion: Greater mortality and higher risk of death if initially observed, trending toward significant
- COMS Small Tumors (1986-1989)
- Prospective. 204 patients, small choroidal melanoms (height 1.0 - 3.0 mm, diameter >= 5.0 mm) not eligible for COMS. Treatment: 8% treated at enrollment, further 33% treated during follow-up. Median F/U 7.7 years
- Outcome; 1997 PMID 9230829 -- "Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group." ([No authors listed], Arch Ophthalmol. 1997 Jul;115(7):886-93.)
- Outcome: 5-year OS 94% (compared with expected general population mortality of 12%; however, patients with serious diseases excluded from COMS), 8-year OS 85%; 22% deaths due to metastatic melanoma and 67% occurred >5 years
- Conclusion: Low risk of dying from small melanoma
- Predictors; 1997 PMID 9400787 -- "Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group." ([No authors listed], Arch Ophthalmol. 1997 Dec;115(12):1537-44.)
- Outcome: growth to be eligible for entry to COMS 2-years 21%, 5-years 31%
- Negative factors: height and diameter, orange pigment, absence of drusen, absence of retinal pigment adjacent to tumor
- Conclusion: ~30% will grow by 5 years
Surgery
[edit | edit source]- Historically the only treatment modality
- Review of data published in 1978 suggested that enucleation may increase risk of metastasis, and subsequent death. Subsequent analyses challenged this observation, but search for alternative treatments was on. Randomized data from COMS Medium show no difference in rate of mets between enucleation and brachytherapy (12-years 17% vs. 21%)
- Treatment approaches:
- Eyewall (trans-scleral or lamellar) tumor resection - May be used in highly selected patients, typically together with adjuvant RT
- Enucleation - Historically gold standard. Indications today include patient request, failure of alternative therapy, tumor >40% intraocular volume, tumor in a blind or painful eye, marked neovascularization. Should not be offered with metastatic disease, unless the eye is painful
- Exenteration - Indication if diffuse extraocular extension, without metastases
- Meta-Analysis; 1992 (1966-1988) PMID 1531290 -- "A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988." (Diener-West M, Arch Ophthalmol. 1992 Feb;110(2):245-50.)
- Pooled analysis. 8 studies that reported 5-year mortality by size
- 5-year survival after enucleation:
- Small tumors: 84%
- Medium tumors: 68%
- Large tumors: 47%
- Review; 1978 PMID 352389 -- "Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells." (Zimmerman LE, Br J Ophthalmol. 1978 Jun;62(6):420-5.)
- Review of survival data suggesting that 1) mortality prior to enucleation is ~1% per year, 2) mortality rises abruptly after enucleation to ~8% during second year, 3) ~2/3 of deaths can be attributed to dissemination of tumor emboli at the time of surgery
Enucleation vs. RT
[edit | edit source]Randomized
- COMS Medium (1987-1998)
- Randomized. 1317 patients. Choroidal melanoma, height 2.5 - 10.0 mm, size <=16.0 mm. Ineligible if contiguous with optic disc. Arm 1) Enucleation vs. Arm 2) I-125 brachytherapy
- Brachytherapy: episcleral plaque, 2-3 mm margin beyond base of tumor (i.e. 4-6 mm larger than maximum basal diameter). If near optic nerve, allowed notches. Dosimetry based on TG43. If tumor height 2.5-4.9 mm, prescription 5 mm from interior surface of sclera; if tumor height >=5 mm, prescription to apex. Dose 85 Gy (dose rate 0.42-1.05 Gy). Organs at risk sclera (1mm from surface of plaque), optic nerve (center of optic disc), macula (foveola), opposite retina (22 mm from scleral surface along diameter of globe)
- 5-years; 2001 PMID 11448319 -- "The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18." (Diener-West M, Arch Ophthalmol. 2001 Jul;119(7):969-82.). Minimum F/U 2 years, 81% F/U 5+ years, 32% F/U 10+ years
- Outcome: 5-year OS enucleation 81% vs. I-125 82% (NS); Risk of death from metastatic disease 11% vs. 9% (NS)
- Predictors: age, height, diameter, distance to optic disc, smoking
- Toxicity: I-125 5-year visual acuity <20/200 63%, <5/200 45%, enucleation 12%
- Conclusion: No difference in mortality between enucleation and I-125 brachytherapy
- Second cancers; 2005 PMID 15883277 -- "Second primary cancers after enrollment in the COMS trials for treatment of choroidal melanoma: COMS Report No. 25." (Diener-West M, Arch Ophthalmol. 2005 May;123(5):601-4.)
- Subset analysis. 2320 patients, routinely followed on protocol
- Outcome: 8% developed second primary (prostate 23%, breast 17%); 51% died (33% with metastatic melanoma)
- RT: No increased risk
- Conclusion: Routine medical surveillance is important
- QOL; 2006 PMID 16476893 -- "Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3." (Melia M, Arch Ophthalmol. 2006 Feb;124(2):226-38.)
- Subset analysis. 209 patients; telephone interview
- Outcome: both groups significantly worse difficulty with vision-oriented activities and bodily/ocular pain. Small differences between arms; brachytherapy better for driving and peripheral vision at 2 years, no difference by 3-5 years after treatment
- Side effect: Brachytherapy worse for anxiety scores
- Conclusion: Brachytherapy better visual function during first 2 years, then no difference, but worse anxiety
- 12-years; 2006 PMID 17159027 -- "The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28." (COMS, Arch Ophthalmol. 2006 Dec;124(12):1684-93.). Minimum F/U 5 years, 39% F/U 12+ years
- Outcome: 12-year OS enucleation 59% vs. I-125 57% (NS); Risk of death from metastatic disease 17% vs. 21%
- Predictors: age, diameter
- Conclusion: No survival difference between enucleation and I-125 brachytherapy
Retrospective
- MGH; 1990 (1965-1984) PMID 2374681 -- "Relative survival rates after alternative therapies for uveal melanoma." (Seddon JM, Ophthalmology. 1990 Jun;97(6):769-77.)
- Retrospective. 1051 patients with uveal melanoma: 556 treated with proton beam (median F/U 5.3 years), compared with 238 patients treated with enucleation (median F/U 8.8 years, same time period), compared with 257 patient enucleated previously (median F/U 17.0 years)
- Outcome: RR for all-cause mortality earlier decade surgery 1.6, current decade surgery 1.2 compared with proton beam; SS at 2 years, no difference after 6 years
- Conclusion: No difference in survival between enucleation and proton beam
- Manhattan Eye & Ear; 1988 PMID 3415941 -- "Cobalt plaque versus enucleation for uveal melanoma: comparison of survival rates." (Adams KS, Br J Ophthalmol. 1988 Jul;72(7):494-7.)
- Retrospective. 639 patients with uveal melanoma; 223 treated with cobalt plaque (median F/U 4.3 years), compared with 416 patients treated with enucleation
- Outcome: No difference in survival
- Predictors: location of tumor, largest tumor dimension
- Conclusion: No difference in survival between enucleation and cobalt plaque
Enucleation +/- neoadjuvant RT
[edit | edit source]- COMS Large (1986-1994)
- Randomized. 1003 patients. Choroidal melanoma, height >2 mm and diameter >=16 mm, or height >=10 mm regardless of diameter, or height >=8 mm and close (<2mm) to optic disc. Ineligible if >=2 mm extrascleral extension. Arm 1) enucleation alone vs. Arm 2) EBRT 20/5 followed by enucleation on last day of treatment. Target choroidal melanoma, posterior half of globe, 1cm margin
- 5-years; 1998 PMID 9645716 -- "The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: initial mortality findings. COMS report no. 10.) ([No authors listed], Am J Ophthalmol. 1998 Jun;125(6):779-96.)
- 5-year outcome: OS enucleation alone 57% vs. RT + enucleation 62% (NS); death from melanoma 28% vs. 26% (NS)
- Prognostic factors: age and diameter
- Conclusion: No survival benefit to neoadjuvant RT using 20/5
- Complications; 1998 PMID 9744369 -- "The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma III: local complications and observations following enucleation COMS report no. 11." ([No authors listed], Am J Ophthalmol. 1998 Sep;126(3):362-72.)
- Complications: Acute complications more with RT (8% vs. 4%), but all minor. 5-year complications: poor prosthetic mobility control 16% vs. RT 19%
- Conclusion: Complications infrequent, minor, and comparable
- 10-years; 2004 PMID 15629284 -- "The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24." (Hawkins BS, Am J Ophthalmol. 2004 Dec;138(6):936-51.). Minimum F/U 5 years
- 10-year outcome: OS 39% both arms (NS); death with melanoma mets RT+surgery 45% vs. surgery alone 40%
- Predictors of death: age >60, diameter >=18 mm. If both, 10-year OS 20%; if neither, OS 58%
- Conclusion: No survival advantage to pre-enucleation RT
Transscleral tumor resection (TTR) vs. brachytherapy
[edit | edit source]Retrospective
- Germany; 2002 PMID 12359606 -- "Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas." (Bechrakis NE, Ophthalmology. 2002 Oct;109(10):1855-61.)
- Retrospective. 152 patients with large uveal melanoma. Treated with transsclear tumor resection or I-125 brachytherapy. Mean height 9.4 mm, mean diameter 14.5 mm, mean distance to optic disc 9.9 mm. Mean F/U 2.3 years
- Outcome: Visual acuity >20/200 TTR 61% vs. I-125 brachytherapy 6% (SS). No difference in eye retention or mortality
- Toxicity: Secondary glaucoma 6% vs. 33% (SS)
- Conclusion: TTR better visual function and less glaucoma than I-125 brachytherapy
Plaque brachytherapy
[edit | edit source]- ABS recommendations; 2003 - PMID 12738332 — "The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas." Nag S et al. Int J Radiat Oncol Biol Phys. 2003 Jun 1;56(2):544-55.
- Patient selection: Small melanoma (<2.5 mm height, <10 mm diameter): observe for growth. Medium melanoma (2.5 - 10 mm height, <16 mm diameter): episcleral plaque, tissue sample not required. Large melanoma (>10 mm height or >16 mm diameter): some patients may be plaque candidates. Not suitable for brachytherapy: gross extrascleral extension, ring melanoma, >50% ciliary body involvement
- Dose: minimum I-125 dose 85 Gy to apex, dose rate 0.6-1.05 Gy/h using TG-43 (assumes point source approximation, no anisotropy corrections, no side attenuation or backscatter from shield, and Silastic insert)
- Guidelines specified for patient selection, dose, isotope selection, prescription, plaque design and fabrication, and complication
Isotope considerations
- I-125: standard, low-energy, was mandated in COMS trials
- Pd-103: lower energy than I-125, with faster dose fall-off. Seeds equivalent size as I-125
- Ru-106: even more rapid dose fall-off, which allows to spare more normal ocular structures. Minimal scleral radiation dose of 300–400 Gy and a minimal apex dose of 80–100 Gy to the tumor apex is reasonable for cure. Parameters can be met if tumor apex <= 5 mm, good choice for small melanomas. Bertil Damato;2005 PMID 15913907 — "Local tumor control after Ru-106 brachytherapy of choroidal melanoms" Damato B. Int. J. Radiation Oncology Biol. Phys., Vol. 63, No. 2, pp. 385–391, 2005.
- Co-60: high-energy gamma, which may result large dose to unaffected ocular structures, and health care providers
- Ir-192: same as Co-60
Visual complications
- Thomas Jefferson; 2000 PMID 10980767 — "Plaque radiotherapy for uveal melanoma: long-term visual outcome in 1106 consecutive patients." Shields CL. Arch Ophthalmol. 2000 Sep;118(9):1219-28.
- 1106 pts w/ upfront visual acuity better than 20/100 tracked for change in vision. Loss of visual acuity (<20/200) seen in 34% at 5 yrs and 68% at 10 yrs.
- Factors predicting poor visual acuity included increasing tumor thickness, proximity to foveola <5 mm, patients > 60 y.o, recurrence, and cobalt.
- Conclusions: Visual acuity best preserved for small tumors >5 mm from foveola/optic disk, and w/ I-125.
Macular Edema
- May be seen as early as 4 months after plaque RT, with peak incidence 12-18 months
- Associated with significant vision loss
- Randomized trial from Thomas Jefferson shows significant benefit for periocular triamcinolone
- Thomas Jefferson (2005-2005) -- Plaque RT +/- periocular triamcinolone
- Randomized. 163 patients, new diagnosed uveal melanoma, I-125 plaque brachytherapy. Arm 1) control vs. Arm 2) periocular injection of triamcinolone acetonide (40 mg in 1 ml) at time of plaque insertion, 4 months, and 8 months later. Optical coherence tomography
- 2009 PMID 19481812 -- "Periocular Triamcinolone for Prevention of Macular Edema after Plaque Radiotherapy of Uveal Melanoma A Randomized Controlled Trial." (Horgan N, Ophthalmology. 2009 May 28.) Mean F/U >1.5 years
- Outcome: Macular edema control 58% vs. triamcinolone 36% (SS). NNT 4.5 patients. Moderate vision loss 48% vs. 31% (SS), severe vision loss 15% vs. 5% (SS)
- Toxicity: elevated IOP (NS), cataract progression (NS)
- Periocular triamcinolone beneficial in reducing risk of macular edema, and moderate/severe vision loss
Gamma Knife
[edit | edit source]- Tufts; 2009 (2000-2007) PMID 19286331 -- "Dose de-escalation with gamma knife radiosurgery in the treatment of choroidal melanoma." (Schirmer CM, Int J Radiat Oncol Biol Phys. 2009 Sep 1;75(1):170-6. Epub 2009 Mar 13.)
- Retrospective. 14 patients, treated with SRS. Mean marginal dose 22 Gy (20-25). Mean tumor volume 1.1 cc
- Outcome: Local control 93%. Intraocular and DM spread in 1 patient
- Toxicity: Visual preservation in 36%
- Conclusion: Choroidal melanoma can be safely treated with marginal dose <25 Gy
Proton Therapy
[edit | edit source]- Nice Hospital; 2010 (1991-2007) PMID 19910136 -- "Proton beam radiotherapy for uveal melanomas at nice teaching hospital: 16 years' experience." (Caujolle JP, Int J Radiat Oncol Biol Phys. 2010 Sep 1;78(1):98-103. Epub 2009 Nov 10.)
- Retrospective. 886 patients, uveal melanoma T1 4%, T2 47%, T3 46%, T4 2%. Median F/U 5.3 years
- Outcome: Local control 5-years 94%, 10-years 92%. 5-year OS T1 92%, T2 89%, T3 67%, T4 62%; 10-year OS 86%, 78%, 43%, 41%. Ocular preservation 5-years 91%, 10-year 87%. Predictors for death: higher age, higher thickness, larger basal diameter, higher volume, and higher volume/eyeball ratio
- Conclusion: Outcomes reported
- Harvard (1989-1998) -- Protons 70 CGE vs Protons 50 CGE
- Randomized. 188 patients with small/medium choroidal melanoma (<15mm diameter, <5mm height), within 4 disc diameters of optic disc or macula. Arm 1) 50 CGE vs. Arm 2) 70 CGE
- 2000 PMID 10865313 -- "A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma." (Gragoudas ES, Arch Ophthalmol. 2000 Jun;118(6):773-8.)
- 5-year outcome: visual acuity >20/200 similar at 55%; tumor regrowth similar at ~3%; mets similar at ~8%
- Toxicity: 50 CGE significantly less visual loss; no difference in radiation papillopathy or maculopathy
- Conclusion: Lower dose resulted in similar outcome, similar visual acuity loss, but improved visual field loss
Heavy Ions
[edit | edit source]- UCSF; 1993 PMID 8414414 -- "Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial." (Char DH, Ophthalmology. 1993 Oct;100(10):1547-54.)
- Randomized. 184 patients, diameter <15mm, thickness <10 mm. Arm 1) helium ion with vs. Arm 2) I-125 plaque brachytherapy. Dose 70 Gy in both arms, but significantly different in delivery: Helium ion given 70/5 over 2 minutes each vs. I-125 continuous LDR 0.7-0.75 Gy /hr
- Outcome: local failure He 0% vs. 13%; enucleation 2x more common after plaque. No difference in survival
- Toxicity: More anterior segment complications after He.
- Conclusion: RT can be used to manage uveal melanoma, with eye retention. Better control with He ion
- Comment: Results led to increase in dose and dose-rate of plaque treatments
Treatment Toxicity
[edit | edit source]- Thomas Jefferson; 1999 PMID 10037576 -- "Early-onset scleral necrosis after iodine I 125 plaque radiotherapy for ciliochoroidal melanoma." (Correa ZM, Arch Ophthalmol. 1999 Feb;117(2):259-61.)
- Case report. 62 M with ciliochoroidal melanoma, treated with I-125 plaque
- Outcome: scleral necrosis with tumor extrusion within 1 month of treatment
Salvage Radiation
[edit | edit source]Proton Therapy
[edit | edit source]- Harvard; 2010 (1961-2000) PMID 20472356 -- "Uveal Melanoma Recurrence After Fractionated Proton Beam Therapy: Comparison of Survival in Patients Treated With Reirradiation or With Enucleation." (Marucci L, Int J Radiat Oncol Biol Phys. 2010 May 14. [Epub ahead of print])
- Retrospective. 73 patients with recurrence after proton beam, treated with PT (42%) or enucleation (58%). Enucleation for larger tumors, no difference in location or ciliary body involvement. Median F/U 5 years
- Outcome: Median OS PT 7.5 years vs enucleation 3.5 years, 5-year OS 63% vs 36% (SS), DM 34% vs 69% (SS)
- Conclusion: Survival in patients with recurrence not compromised by second course of PT
