Radioactive Waste Management/Spent Nuclear Fuel

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant) to the point where it is no longer useful in sustaining a nuclear reaction.
After uranium fuel has been used in a reactor for a while, it is no longer as efficient in splitting its atoms and producing heat to make electricity. It is then called “spent” nuclear fuel. About one-fourth to one-third of the total fuel load is spent and is removed from the reactor every 12 to 18 months and replaced with fresh fuel. The spent nuclear fuel is high-level radioactive waste.
The NRC regulates all commercial reactors in the United States, including nuclear power plants that produce electricity, and university research reactors. The agency regulates the possession, transportation, storage and disposal of spent fuel produced by the nuclear reactors.
Spent nuclear fuel is highly radioactive and potentially very harmful. Standing near unshielded spent fuel could be fatal due to the high radiation levels. Ten years after removal of spent fuel from a reactor, the radiation dose 1 meter away from a typical spent fuel assembly exceeds 20,000 rems per hour. A dose of 5,000 rems would be expected to cause immediate incapacitation and death within one week.
Some of the radioactive elements in spent fuel have short half-lives (for example, iodine-131 has an 8-day half-life) and therefore their radioactivity decreases rapidly. However, many of the radioactive elements in spent fuel have long half-lives. For example, plutonium-239 has a half-life of 24,000 years, and plutonium-240 has a half-life of 6,800 years. Because it contains these long half-lived radioactive elements, spent fuel must be isolated and controlled for thousands of years. A second hazard of spent fuel, in addition to high radiation levels, is the extremely remote possibility of an accidental “criticality,” or self-sustained fissioning and splitting of the atoms of uranium and plutonium.
NRC regulations therefore require stringent design, testing and monitoring in the handling and storage of spent fuel to ensure that the risk of this type of accident is extremely unlikely. For example, special control materials (usually boron) are placed in spent fuel containers to prevent a criticality from occurring. Nuclear engineers and physicists carefully analyze and monitor the conditions of handling and storage of spent fuel to guard further against an accident. A barrier or radiation protection shield must always be placed between spent nuclear fuel and human beings. Water, concrete, lead, steel, depleted uranium or other suitable materials calculated to be sufficiently protective by trained engineers and health physicists, and verified by radiation measurements, are typically used as radiation shielding for spent nuclear fuel.
Spent fuel may be stored in either a wet or dry environment. In addition, it may be stored either at the reactor where it was used or away from the reactor at another site. The various techniques are as follows:
Wet Storage
[edit | edit source]Currently most spent nuclear fuel is safely stored in specially designed pools at individual reactor sites around the country. The water-pool option involves storing spent fuel in rods under at least 20 feet of water, which provides adequate shielding from the radiation for anyone near the pool. Most spent fuel from nuclear power plants is stored under water, as shown at the Diablo Canyon plant in California. The rods are moved into the water pools from the reactor along the bottom of water canals, so that the spent fuel always is shielded to protect workers.
A typical spent fuel rod is about 12 feet long and 3/4 inch in diameter. The rods are arranged in somewhat square arrays, known as fuel assemblies, that range in size from an array of 6 rods by 6 rods to an array of 17 rods by 17 rods. The fuel pools vary in size from a capacity of 216 to 8,083 fuel assemblies. Most pools were originally designed to store several years worth of spent fuel. Due to delays in developing disposal facilities for the spent fuel, licensees have redesigned and rebuilt equipment in the pools over the years to allow a greater number of spent fuel rods to be stored. However, this storage option is limited by the size of the spent fuel pool and the need to keep individual fuel rods from getting too close to other rods and initiating a criticality or nuclear reaction.
Dry Storage
[edit | edit source]If pool capacity is reached, licensees may move toward use of above-ground dry storage casks. The first dry storage installation was licensed by the NRC in 1986. In this method, spent fuel is surrounded by inert gas inside a container called a cask. The casks can be made of metal or concrete, and some can be used for both storage and transportation. They are either placed horizontally or stand vertically on a concrete pad. Seventeen nuclear power plants are currently storing spent fuel under the dry storage option.
Spent fuel may be stored in dry casks either horizontally, as shown at the H.B. Robinson nuclear power plant in South Carolina, or vertically, as shown at the Surry nuclear power plant in Virginia.
Away-from-Reactor Storage
[edit | edit source]General Electric Company has a facility to store spent fuel away from reactors, using the wet storage pool technology, at Morris, Illinois. GE received a license to receive and store nuclear material at this facility in 1971. The facility is essentially full, and the company has completed contracts with specific utilities (under which it had agreed to accept their used fuel) and has no plans to accept additional spent fuel.
Storage Differences
[edit | edit source]Both pool storage and dry storage are safe methods, but there are significant differences. Pool storage requires a greater and more consistent operational vigilance on the part of utilities or other licensees and the satisfactory performance of many mechanical systems using pumps, piping and instrumentation. Dry storage, which is almost completely passive, is simpler, uses fewer support systems and offers fewer opportunities for things to go wrong through human or mechanical error. Dry storage is not suitable for fuel until the fuel has been out of the reactor for a few years and the amount of heat generated by radioactive decay has been reduced. Monitored Retrievable Storage The Nuclear Waste Policy Act (NWPA) of 1982 authorized the Department of Energy (DOE) to construct a monitored retrievable storage (MRS) facility for storage of high-level waste, with certain restrictions.
Representatives of state and local governments and Indian
tribes and members of the public would be invited to participate
in meetings on an MRS facility.
NRC would publish notice of receipt of DOE’s application to
build an MRS facility and hold a public hearing, if requested,
before issuance of the license.
About 160,000 spent fuel assemblies, containing 45,000 tons of spent fuel from nuclear power plants, are currently in storage in the United States. Of these, about 156,500 assemblies are stored at nuclear power plants, and approximately 3,500 assemblies are stored at away-from-reactor storage facilities, such as the General Electric plant at Morris, Illinois. The vast majority of the assemblies are stored in water pools, and less than 5% are stored in dry casks. About 7,800 used fuel assemblies are taken out of reactors each year and are stored until a disposal facility becomes available.
If all the 160,000 spent fuel assemblies currently in storage were assembled in one place, they would only cover a football field about 5 1/2 yards high.
DOE is developing plans for a permanent disposal facility for spent fuel from nuclear power plants (as well as for the highlevel waste that has been produced by the nation’s nuclear weapons production activities).
Congress has directed DOE to focus on a proposed site at Yucca Mountain, Nevada, for the disposal facility. This has aroused some controversy, particularly with state and local authorities.
Studies are still underway to determine if the site is adequate for permanent disposal of the high-level waste. NRC has a rigorous regulatory program for review of these ongoing DOE site investigations.
DOE would design, build and operate the facility, subject to federal regulations and oversight by the NRC. The NRC must approve the site and design for the disposal facility, as well as inspect it during construction and operation.
Once DOE submits an application to construct a repository, the NWPA calls for NRC to complete its review within three years.
If the NRC authorizes construction, DOE will proceed with constructing the repository and would submit a license application update (containing additional details on design and construction of the facility) to the NRC. This would be followed by an NRC decision on whether to license operation of the repository.
The Nuclear Waste Policy Act directed the Department of Energy to study Yucca Mountain, Nevada, to determine whether it would be suitable for disposal of high-level radioactive waste. High-Level Radioactive Waste
As required by the NWPA, the NRC has issued technical requirements and criteria for approving or disapproving DOE’s application. These are contained in Part 60 of the NRC’s regulations. Examples include:
- Radiation doses during repository operations must be kept
below regulatory limits. These limits are 100 millirems per year for members of the general public (which is about a third of the average American’s annual dose from nature) and 5,000 millirems per year for workers.
- Waste must be retrievable for 50 years after waste emplacement
begins.
- The container in which the high-level waste will be placed
must maintain its integrity for 300 to 1,000 years.
- The waste packages must not contain explosive or flammable
materials or liquids that could endanger the repository.
Public Involvement
[edit | edit source]Representatives of state and local governments and Indian tribes are invited to participate in meetings on the high-level waste repository. Members of the public may attend as observers. NRC will publish notice of receipt of DOE’s application to build a repository and hold a public hearing before issuance of the construction authorization. When DOE submits an application to receive and possess high-level waste at the LM-300 drill rig at Yucca Mountain, Nevada, obtained underground rock and soil samples that scientists examined to help determine site suitability for high-level waste disposal facility, NRC will again announce receipt of the application and will publish notice of the opportunity for an optional additional public hearing.
Nature of spent fuel
[edit | edit source]Nanomaterial properties
[edit | edit source]Spent enriched uranium|low enriched uranium nuclear fuel is an example of a nanomaterial that existed before the term nano became fashionable. In the oxide fuel, intense temperature gradients exist which cause fission products to migrate. The zirconium tends to move to the centre of the fuel Pelletizing|pellet where the temperature is highest, while the lower-boiling fission products move to the edge of the pellet. The pellet is likely to contain lots of small liquid bubble|bubble-like pores which form during use; the fission xenon migrates to these voids. Some of this xenon will then decay to form caesium, hence many of these bubbles contain a large concentration of 137Cs.
In the case of the MOX the xenon tended to diffuse out of the plutonium-rich areas of the fuel, and it was then trapped in the surrounding uranium dioxide. The neodymium tended to not be mobile.
Also metallic particles of an alloy of Mo-Tc-Ru-Pd tend to form in the fuel. Other solids form at the boundary between the uranium dioxide grains, but the majority of the fission products remain in the uranium dioxide as solid solutions. A paper describing a method of making a non-radioactive "uranium active" simulation of spent oxide fuel exists.[1]
Fission products
[edit | edit source]3% of the mass consists of fission products of 235U and 239Pu (also indirect products in the decay chain); these are considered radioactive waste or may be separated further for various industrial and medical uses. The fission products include every element from zinc through to the lanthanides; much of the fission yield is concentrated in two peaks, one in the second transition row (Zirconium|Zr, Mo, Tc, Ruthenium|Ru, Rhodium|Rh, Palladium|Pd, Silver|Ag) and the other later in the periodic table (Iodine|I, Xenon|Xe, Caesium|Cs, Barium|Ba, Lanthanum|La, Cerium|Ce, Nd). Many of the fission products are either non-radioactive or only short-lived radioisotopes. But a considerable number are medium to long-lived radioisotopes such as 90Sr, 137Cs, 99Tc and 129I. Research has been conducted by several different countries into segregating the rare isotopes in fission waste including the "fission platinoids" (Ru, Rh, Pd) and silver (Ag) as a way of offsetting the cost of reprocessing; however, this is not currently being done commercially.
The fission products can modify the thermal conductivity|thermal properties of the uranium dioxide; the lanthanide oxides tend to lower the thermal conductivity of the fuel, while the metallic nanoparticles slightly increase the thermal conductivity of the fuel.[2]
Table of chemical data
[edit | edit source]| Element | Gas | Metal | Oxide | Solid solution |
|---|---|---|---|---|
| Br krypton|Kr | Yes | - | - | - |
| Rb | Yes | - | Yes | - |
| Sr | - | - | Yes | Yes |
| Y | - | - | - | Yes |
| Zr | - | - | Yes | Yes |
| Nb | - | - | Yes | - |
| Mo | - | Yes | Yes | - |
| Tc ruthenium|Ru rhodium|Rh palladium|Pd silver|Ag cadmium|Cd indium|In antimony|Sb | - | Yes | - | - |
| Te | Yes | Yes | Yes | Yes |
| I xenon|Xe | Yes | - | - | - |
| Cs | Yes | - | Yes | - |
| Ba | - | - | Yes | Yes |
| La cerium|Ce praseodymium|Pr neodymium|Nd promethium|Pm samarium|Sm europium|Eu | - | - | - | Yes |
Plutonium
[edit | edit source]
About 1% of the mass is 239Pu and Plutonium 240|240Pu resulting from conversion of 238U, which may be considered either as a useful byproduct, or as dangerous and inconvenient waste. One of the main concerns regarding nuclear proliferation is to prevent this plutonium from being used by states, Non-Proliferation Treaty#First pillar: non-proliferation|other than those already established as nuclear weapons states, to produce nuclear weapons. If the reactor has been used normally, the plutonium is reactor-grade, not weapons-grade: it contains much 240Pu and less than 80% 239Pu, which makes it less suitable, but not impossible, to use in a weapon.[4] If the irradiation period has been short then the plutonium is weapons-grade (more than 80%, up to 93%).
Uranium
[edit | edit source]96% of the mass is the remaining uranium: most of the original 238U and a little 235U. Usually 235U would be less than 0.83% of the mass along with 0.4% 236U.
Reprocessed uranium will contain Uranium-236|236U, which is not found in nature; this is one isotope which can be used as a fingerprint for spent reactor fuel.
If using a thorium fuel to produce fissile U-233, the SNF will have U-233, with a half-life of 159,200 years. This will have an impact on the long-term radioactive decay of the spent fuel. If compared with MOX fuel, the activity around one million years in the cycles with thorium will be higher due to the presence of the not fully decayed U-233.
Minor actinides
[edit | edit source]Traces of the minor actinides are present in spent reactor fuel. These are actinides other than uranium and plutonium and include neptunium, americium and curium. The amount formed depends greatly upon the nature of the fuel used and the conditions under which it was used. For instance, the use of MOX fuel (239Pu in a 238U matrix) is likely to lead to the production of more 241Am and heavier nuclides than a uranium/thorium based fuel (233U in a 232Th matrix).
For natural uranium fuel: Fissile component starts at 0.71% 235U concentration in natural uranium. At discharge, total fissile component is still 0.50% (0.23% 235U, 0.27% fissile 239Pu, 241Pu) Fuel is discharged not because fissile material is fully used-up, but because the neutron poison|neutron-absorbing fission products have built up and the fuel becomes significantly less able to sustain a nuclear reaction.
Some natural uranium fuels use chemically active cladding, such as Magnox, and need to be reprocessed because long-term storage and disposal is difficult.[5]
For highly enriched fuels used in nuclear marine propulsion|marine reactors and research reactors, the isotope inventory will vary based on in-core fuel management and reactor operating conditions.
Fuel composition and long term radioactivity
[edit | edit source]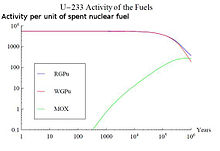

Long-lived radioactive waste from the back end of the fuel cycle is especially relevant when designing a complete waste management plan for SNF. When looking at long-term radioactive decay, the actinides in the SNF have a significant influence due to their characteristically long half-lives. Depending on what a nuclear reactor technology|nuclear reactor is fueled with, the actinide composition in the SNF will be different.
An example of this effect is the use of nuclear fuels with thorium. Th-232 is a fertile material that can undergo a neutron capture reaction and two beta minus decays, resulting in the production of fissile uranium-233|U-233. The SNF of a cycle with thorium will contain U-233, an isotope with a half-life of 160,000 years. Its radioactive decay will strongly influence the long-term radioactive decay|activity curve of the SNF around 1,000,000 years. A comparison of the activity associated to U-233 for three different SNF types can be seen in the figure on the top right.
The burnt fuels are Thorium with Reactor-Grade Plutonium (RGPu), Thorium with Weapons-Grade Plutonium (WGPu) and MOX fuel|Mixed Oxide fuel (MOX). For RGPu and WGPu, the initial amount of U-233 and its decay around 10E5 years can be seen. This has an effect in the total activity curve of the three fuel types. The absence of U-233 and its daughter products in the MOX fuel results in a lower activity in region 3 of the figure on the bottom right, whereas for RGPu and WGPu the curve is maintained higher due to the presence of U-233 that has not fully decayed.
The use of different fuels in nuclear reactors results in different SNF composition, with varying activity curves.
Spent fuel corrosion
[edit | edit source]Noble metal nanoparticles and hydrogen
[edit | edit source]According to the work of the corrosion electrochemistry|electrochemist Shoesmith[6][7] the nanoparticles of Mo-Tc-Ru-Pd have a strong effect on the corrosion of uranium dioxide fuel. For instance his work suggests that when hydrogen (H2) concentration is high (due to the Hypoxia (environmental)|anaerobic corrosion of the steel waste can), the oxidation of hydrogen at the nanoparticles will exert a protective effect on the uranium dioxide. This effect can be thought of as an example of protection by a sacrificial anode, where instead of a metal anode reacting and dissolving it is the hydrogen gas which is consumed.
Disposal of
[edit | edit source]Nuclear reprocessing can separate spent fuel into various combinations of reprocessed uranium, plutonium, minor actinides, fission products, remnants of zirconium or steel cladding, activation products, and the reagents or solidifiers introduced in the reprocessing itself. In this case the volume that needs to be disposed of is greatly reduced.
Alternatively, the intact Spent Nuclear Fuel (SNF) can be disposed of as radioactive waste.
The United States has planned disposal in Deep geological repository|deep geological formations, such as the Yucca Mountain nuclear waste repository, where it has to be shielded and packaged to prevent its migration to mankind's immediate environment for thousands of years. However, on March 5, 2009, United States Secretary of Energy|Energy Secretary Steven Chu told a Senate hearing that "the Yucca Mountain site no longer was viewed as an option for storing reactor waste."
- ↑ "Microstructural features of SIMFUEL - Simulated high-burnup UO2-based nuclear fuel", P.G. Lucuta, R.A. Verrall, Hj. Matzke and B.J. Palmer, Journal of Nuclear Materials, 1991, 178, 48–60.
- ↑ Dong-Joo Kim, Jae-Ho Yang, Jong-Hun Kim, Young-Woo Rhee, Ki-Won Kang, Keon-Sik Kim and Kun-Woo Song, Thermochimica Acta, 2007, 455, 123–128.
- ↑ "Solution of Fission Products in UO2" (PDF). Retrieved 2008-05-18.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ↑ "Additional Information Concerning Underground Nuclear Weapon Test of Reactor-Grade Plutonium". U.S. Department of Energy. Retrieved 2008-05-18.
{{cite web}}: Cite has empty unknown parameter:|month=(help) - ↑ "RWMAC's Advice to Ministers on the Radioactive Waste Implications of Reprocessing". Radioactive Waste Management Advisory Committee (RWMAC). 3 November 2002. Retrieved 2008-05-18.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ↑ "David W. Shoesmith". University of Western Ontario. Retrieved 2008-05-18.
{{cite web}}: Cite has empty unknown parameter:|month=(help) - ↑ "Electrochemistry and corrosion studies at Western". Shoesmith research group, University of Western Ontario. Retrieved 2008-05-18.
{{cite web}}: Cite has empty unknown parameter:|month=(help)
