Semiconductor Electronics/Types of Materials
Matter and Electricity
[edit | edit source]Insulators
[edit | edit source]Insulators are those materials that don't conduct electricity under normal conditions. For instance: air is an insulator. You don't get electrocuted when approaching a plug point since air is an insulator. In abnormal conditions, air behaves like a conductor. For example, during a thunderstorm, extremely high voltage create lightning bolts, which jump from one cloud to another or to the ground. At these extreme situations, air will act as a conductor.
Conductors
[edit | edit source]Conductors are substances that easily let electrical charge pass through them with relatively very-less resistance. Almost all metals are conductors. Whenever you cut an electric wire, you can see copper or some other metal at its core. This is because these metals can easily conduct electricity. Silver can conduct electricity better than copper and copper can conduct electricity better than gold. We don't use them because they are extremely expensive due to their rarity. In cases where electrical circuit needs to be short, like in PCBs and in ICs, gold has been used. Gold is preferred over silver because it's inert and doesn't react and/or corrode as quickly as silver.
Semiconductors
[edit | edit source]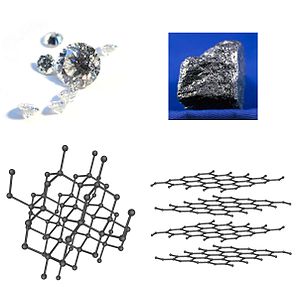
Semiconductors are materials that behave like both conductors and insulators. In certain condition a semiconductor acts like a conductor. For example charcoal and graphite which are allotrope of Carbon act as conductor, as opposed to diamond, which has a different crystal structure and acts as an insulator. The conductivity of semiconductors can be increased by adding certain impurities to them; this is called doping. The conductivity may increase by pumping energy into semiconductor crystal in the form of heat or light.
Science Behind Conduction
[edit | edit source]Atom of an Insulator
[edit | edit source]All atom has a central nucleus which a heavy positively charged mass with relatively small size and located at the center of the atom. The negatively charged electrons go around the nucleus in a path which we call orbit. This is very similar to our solar system. We have Sun, a heavy mass at the center and planets, comets and asteroids go around the Sun. This model was proposed by Niels Bohr, and is called Bohr's atomic model.
It's wrong to think that an orbit is a single well defined path. Instead, each orbit consists of many sub orbitals. This is shown in the diagram below. The outer most orbit of an atom can be divided into various sub orbitals or bands. As you can see below, it has been divided into three colors. The lower most band is called a valence band. The electrons in this band determine the valency of the atom. The upper most band is called the conduction band. In this band, the electron is farthest from the positively charged nucleus and is capable of easily escaping out of the atom and thus enabling the material/atom to conduct electricity.
Normally, an insulator atom does not conduct electricity because there is a forbidden energy gap between the valence and conduction band. The electron has to over come the forbidden energy gap in order for it to orbit in conduction band and conduct electricity. In insulators the forbidden energy gap is very huge, and hence an insulator does not conduct electricity under normal conditions.
However if we can ap an insulator atom with enough power, it can conduct electricity. For example air is an insulator. But during thunder storm lightning bolts pass through air. This is because, the potential difference between two clouds or between the cloud and the ground is enough to make electrons in Nitrogen and Oxygen atoms in air to jump from valence to conduction band. This makes air an temporary conductor till the lightning bolt strikes its destination.
Atom of a Conductor
[edit | edit source]Below you can see the diagram of an atom of an conductor. Here you can notice that there is no forbidden energy gap. The valence and conduction band are overlapping each other. Hence it's extremely easy for electrons to move from valence to conduction band. Hence conductors easily conduct electricity without much loss of energy. They conduct electricity under normal conditions.
Atom of Semiconductor
[edit | edit source]In a semiconductor the forbidden energy gap is neither too high nor two low; for example bandgap of Si is 1.1 eV, Ge is 1.43 eV. In normal conditions some atoms of a semiconductor conduct while other behave like an insulator. This is because there is non homogeneous energy distribution among atoms. Certain atoms have enough energy for their electrons to orbit in conduction band whereas others don't have enough hence their electrons stay in the valence band.
By pumping a little amount of energy or by adding certain impurities to an semiconductor crystal we can make it behave like a conductor. Its these properties of a semiconductor that makes it highly useful for electronics which you will understand as you read this book further and further.
Group IV (four) of the periodic table namely atoms like Carbon, Silicon, Germanium come under semiconductor class.


