Structural Biochemistry/Actin Assembly during Endocytosis

Actin Microfilaments
[edit | edit source]Actin is a protein complex that forms the majority of a cell’s cytoskeleton. The globular portions of action (G-actin) are bound together in the long, thin microfilaments of F-actin. The F-actin microfilaments are double-stranded in a double helix formation. It is the actin-related protein (Arp2/3) complex that facilitates a process called nucleation, which is the formation of a new actin microfilament branch. The daughter filament stems from the parent filament in a y-shape at an expected angle of about 70 degrees. Actin has been observed to have many different functions like muscle contraction, maintaining cell shape, mobility of the cell, division of the cell and also in cell signaling. The function of interest here is the role of actin in the many steps of endocytosis.
Endocytosis
[edit | edit source]Endocytosis is the method by which cells uptake materials by engulfing them with a vesicle made from the cell membrane itself. There are different kinds of endocytosis: clatharin-mediated, alveolae, macropinocytosis, and phagocytosis. The best understood method in mammals is clatharin-mediated endocytosis (CME). Clatharin is a protein component in the cytosol, which helps to form a coated pit on the inner side of the cell membrane. This pit buds into a Clatharin-Coated Vesicle (CCV) that is characteristic of this form of endocytosis. In mammals, it is understood that actin plays a role in this process by helping to pinch off the neck of the vesicle during CME.
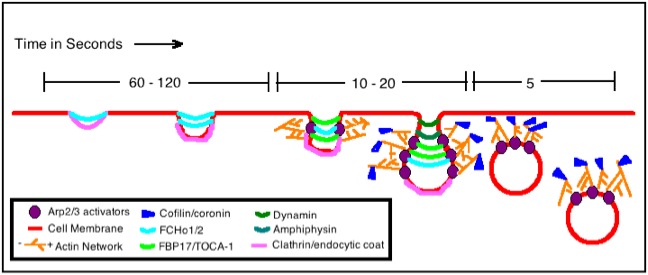
Actin Assembly Model for Endocytosis in Mammalian Cells
[edit | edit source]The figure shows a basic actin assembly model that is particular to helping the endocytosis of mammalian cells, although the model for yeast actin assembly is quite similar. In yeast it has been proven that actin is always required for endocytosis, but this mammalian endocytosis only needs actin under certain conditions: for instance when the thing being ingested by the cell is very large or when the location of endocytosis is already rich with actin microfilaments.
This specific model is for actin assembly during the process of Clathrin-Mediated Endocytosis. The clathrin and other specific adaptors (FCHo1/2 and FBP17/TOCA-1) are the first to start the curvature of the cell membrane in this process. Then the compound Arp2/3 is introduced, whose purpose is the regulation of actin filament nucleation. The actin filaments continue to grow longer through polymerization and this process at the barbed ends of actin helps to force the neck of the vesicle to elongate. The entire infolding of the vesicle gets longer and eventually membrane fission occurs with the help of additional amphipysins and dynamin. Lastly, the proteins cofflin and coronin are employed to dissemble the actin filaments once the job of endocytosis is completed and the vesicle is well within the cell. The entire process of the cell membrane invagination, elongation and fission in the process of endocytosis is mediated by the formation and subsequent pushing actions of these actin filaments.
Advancing our understanding of the Endocytic Process
[edit | edit source]Improvements in technology and methodology using the light and electron microscope have been significant in advancing the understanding of molecular mechanisms. Fluorescent proteins especially green fluorescent proteinss (GFPs) have allowed the individual components to be studied and follow the movement of endocytic structures. The development of pHluorin as a GFP derivative is sensitive in luminescence with pH levels. It is a useful tool to mark the time of scission of the endocytic vesicle from the plasma membrane. Great improvements in signal-to-noise rations have been provided by the development of confocal microscopy, both laser scanning and spinning disk, and by implementation of total internal reflection fluorescence microscopy. This has improved the visualization of faint fluorescent signals. There have been advances in electron microscopy especially in the ability to visualize clathrin assemblies and actin filaments. This has benefited from the use of freeze-etch, platinum replica, and tomographic approaches. Quantitative measurements of GFP fluorescence can be converted to absolute numbers of molecules by comparison with standards in cells. Computers track these individual entities such as endocytic vesicles and clathrin-rich sites providing data on their fluorescence intensity. Computers have given the ability to analyze hundreds to thousands of these entities.
Future Studies
[edit | edit source]Most of our knowledge of the role of actin in the process of endocytosis comes from the exploration of this process in yeast and not in mammals. Thusly the process in mammals and the true nature of the actin microfilaments are largely unknown. Some important further issues arise, such as determining exactly where and how the actin creates the force to cause vesicle fission at the neck of the invagination. Another potential field of interest is discovering why actin is not always necessary for the endocytic process in mammals. It would be useful to know how the cell signals its need for actin and the exact signaling process that leads to this actin assembly. Overall, the study of this process must continue to take place more in mammalian cells in order to gain more knowledge of actin’s functioning in the cell.
References
[edit | edit source]Mooren, Olivia L., Brian J. Galletta, and John A. Cooper. "Roles for Actin Assembly in Endocytosis." Annual Review of Biochemistry (2012): 661-86. Biochemistry. Annual Reviews, 2012. Web. 6 Dec. 2012. <http://ucelinks.cdlib.org:8888/sfx_local?sid=Entrez:PubMed&id=pmid:22663081>.
Akin , O. and Mullins , R. D. Capping Protein Increases the Rate of Actin-Based Motility by Promoting Filament Nucleation by the Arp2/3 Complex. Cell 133, 841–851 (2008). doi: 10.1016/j.cell.2008.04.011
McPherson PS, Ritter B, Wendland B. Clathrin-Mediated Endocytosis. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-. Available from: http://www.ncbi.nlm.nih.gov/books/NBK6479/