Structural Biochemistry/Caffeine


Caffeine is the most popular drug that is being used to maintain a certain mental stability such as staying awake, changing the way the brain functions, and moods. It is found in many of the drinks we consume daily. This includes, but not limited to sodas, coffee, and tea. Many consume this drug without even being conscious of their daily intake, and it is not something that most people think of as being dangerous or questionable. Although Caffeine is taken very lightly, large amounts of this product are in many of our drinks. For example, according to an article, "Neuropsychiatric Effects of Caffeine", by Anthony Winston, it shows that 100 mg is in a normal cup of coffee, 75 mg in instant coffee, and 50 mg in tea that we drink every day (Winston, page 432).
Commonly, caffeine starts to affect the brain and body within an hour and begins to wear off after 3 to 4 hours.
Caffeine is mainly use for the purpose to raise and boost mental functions, but when it is overly used, it can also advance into a harmful state called caffeinism. According to Winston, “caffeine is characterized by restlessness, agitation, excitement, rambling thought, speech, and insomnia" (page 1). Following these symptoms, many other disorders such as anxiety, sleep, eating disorders, and many more may occur.
Caffeine (1, 3, 7-trimethylxanthine) is a natural product found in plants. While the actual caffeine content of plant seeds and leaves varies quite a bit from species to species, caffeine is viewed as the most abundant naturally-occurring purine alkaloid, meaning it is derived from one or more purine nucleotides. Alkaloids are a large group of compounds that can be found mainly in plants and contain basic nitrogen atoms. These compounds usually exist as salts because of their basic nature. Major caffeine sources include the seeds of the coffee plant (Coffea Arabica), tea leaves (Camellia Sinensis), and cola nuts. It is widely accepted that caffeine is a stimulant when consumed by humans, with its main focus falling upon the central nervous system.[1] Plants utilize caffeine as a line of defense. It works as an insecticide for the plant, deterring many potential threats as it can obstruct metabolic pathways.[2]
Effects of Caffeine: How and Why?
[edit | edit source]By in-taking caffeine, the body feels a sense of alertness and wakefulness. The reason caffeine affects the body in the way that it does is because of its structural resemblance to both adenosine and cyclic adenosine monophosphate (cAMP). As a result of similar structures, caffeine can potentially bind to receptors that usually bind to adenosine and its derivatives. Within the body, adenosine plays a role in regulating the brain and its activity. When there are large buildups of adenosine in the brain, they begin to bind to the brain receptors and that causes reactions leading to sleepiness and feeling drowsy. However, when caffeine enters the body it binds to these same receptors because of its similar structure and this prevents adenosine from binding to these receptors and this delays the body from feeling sleepy.

cAMP is a secondary messenger that is responsible for processes involving blood pressure and oxygen in the body. It increases the amount of oxygen delivered to the brain as well as blood pressure, both of which keep the body alert. However, there is an enzyme found inside the body, cyclic nucleotide phosphodiesterase (cAMP-PDE), that breaks down cAMP and removes its anti-drowsy effects from the body. As a result of its similarity to the structure of cAMP, caffeine prevents cAMP-PDE from breaking down cAMP and allows the anti-drowsy effects to prolong for much longer.

Disorders
[edit | edit source]
- Sleep Disorders:
Insomnia is sometimes the outcome of an excessive amount of caffeine. When taken near bed time, it may extend the hours awake and give the inability to easily fall asleep. In addition, according to Winston, "it reduces slow-wave sleep in the early part of the sleep cycle and can reduce REM sleep later in the cycle" (page 2). In conclusion, wakefulness is a symptom of the sleeping disorder caused by caffeine if taken inconsiderably.
- Eating Disorders:
Caffeine is also a cause of Anorexia and Bulimia nervosa. Studies have shown that people with these eating disorders frequently have an extensive intake of caffeine. This drug gives way to a smaller appetite and a fast metabolism. In addition, it may also be dangerous for the heart as well as an increase in chance of receiving osteoporosis.
Synthesis
[edit | edit source]In this synthesis, "SAM" refers to S adenosyl-L-methionine, which causes the addition of a methyl group.[2]

Coffee and linkage to weight loss: Caffeine is a stimulant that stimulates the central nervous system, helps when dealing with fatigue, aids in preventing sleepiness and tiredness, and improves with alertness, mood, metabolism. It is important that caffeine is monitored and taken at a balanced diet. A common source of caffeine is coffee. About two to four cups of coffee a day is considered healthy. Energy drinks and caffeine-carbonated drinks contain a dangerous amount of caffeine. Drinking more than two a day is enough to do its intended job. Any more can cause serious problems. Caffeine is also linked to weight lost. Caffeine may help shed a few pounds, or block further weight gain. Caffeine is one of the ingredients in the dangerous weight loss pills that people depend on to lose weight. Caffeine is thought to have contribute to weight loss due to the fact that it can: suppress appetite, increase metabolism and chances of burning calories, and induce water weight loss. Caffeine may suppress appetite for a short period of time, however it seems to not have any correlation with suppressing appetite for a long period of time. Caffeine may implement and induce thermogenesis, which is the body's way of creating heat while digesting food. Caffeine may also present the body with alertness and behavioral changes, which may help during exercise and induce faster calorie burning. Caffeine burns fat, which preserves the alternative (glycogen, glucose, amino acids) from being burned. This keeps the sugar in the bloodstream, and enables the body to run a little longer without getting hungry. Caffeine has also been a popular choice of intake before a workout. If someone intakes caffeine before a workout, it is shown that caffeine isolates extra fat flowing, and targets and accesses the fat for energy, rather than carbs or existing muscle. Caffeine targets and breaks the fat, and is rapidly burned at that instant. However, it is important to drink a lot of water during the workout to prevent dehydration. Caffeine, coffee in particular, may lead to eating disorders, such as anorexia and bulimia, because of the notion that coffee has 0 calories and 0 carbs. Coffee indeed has 0.5-0.9g of carbs. Coffee may also help a person reduce the cravings and need for food, with its bitter taste, and convince someone that he or she is not hungry. Caffeine is also known to prevent fat from building and collecting in cells. There is research on the caffeine inhibiting enzymes, and its role in fat synthesis. Caffeine is the main ingredient in numerous diet pills. People with eating disorders turn to caffeine and coffee for energy and satisfaction of hunger. However, in this case, it is more harmful than effective. Caffeine can also increase urination since it is a diuretic. An increased urination may help shed water weight and bloatedness. However, losing weight through the removal of fluids will be effective for only a short period of time. Caffeine is not intended to serve as a meal replacement, or as a sole source of weight loss. Relying on caffeine to lose weight is not a healthy or efficient method. Since the thermogenetic characteristic of caffeine is one adopted by weight loss drugs, caffeine can be chosen as an alternative to acquire thermogenesis, rather than from weight loss drugs itself. Relying on caffeine can cause extreme health effects, both physically and mentally. It can increase chances of insomnia, nervousness, makes a person jittery, instability, rapider heart rate, and potassium deficiency. Pregnant women should prevent intaking caffeine at a large rate, since caffeine runs through the bloodstream and directly to the fetus. It is important to take into consideration that in order to have an efficient weight loss, diet and exercise are the two most crucial aspects and factors. Relying and overdosing on caffeine will have damaging effects and may cause the body to gain weight, instead of intended loss.
Extraction
[edit | edit source]Tea Leaves
[edit | edit source]Caffeine can be extracted and isolated from tea leaves. Since caffeine is soluble in both water and organic solvents, it can be first extracted from the leaves through solid-liquid extraction in hot water and then separated even further through extraction with a polar non-protic organic solvent such as methylene chloride. Before beginning the extraction process, the components of tea leaves should be analyzed:
- Cellulose: Cellulose functions as a rigid and insoluble structural component and makes up a large portion of leaves. This linear polymer is made up of D-glucopyranose units connected between carbons 1 and 4. Because of its high molecular weight, cellulose is not soluble in water despite having hydroxyl groups.

Cellulose Sessel
~In a solid liquid extraction of tea with hot water, the cellulose would be easy to remove because as the caffeine is extracted into the water, the cellulose component is left behind. The cellulose remains in the tea leaf while the caffeine and the other water-soluble components are extracted.
- Proteins and Pigments: Both pigments and proteins are highly soluble in water and therefore do not pose a problem to the extraction of caffeine. In the initial solid-liquid extraction, the proteins and pigments are extracted along with the caffeine. But since they are non polar, the second extraction of caffeine into methylene chloride, which is immiscible in water, results in the extraction of the caffeine without extracting the proteins and pigments. These stay in the aqueous phase while the caffeine is extracted into this water immiscible methylene chloride.
- Tannins: Tannins are polyphenolic compounds with variable molecular weights and are the reason why tea has a bitter taste. The compounds are made up of D-glucose units that have ester bonded to units of Gallic Acid. Tannins do pose a problem to the extraction of caffeine because it is soluble in both water and organic solvents. However, this problem can be solved by cleaving the ester bonds with a weak base, such as Calcium Carbonate. This causes the tannins to break down into glucose and calcium salt of Gallic Acid, both of which will not be extracted into the organic solvent.
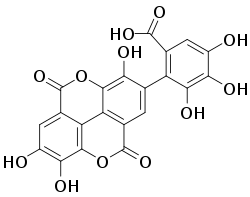
Flavogallonic acid dilactone - Saponins: Saponins are compounds that have both polar and non-polar characteristics. This duality complicates the extraction process because organic compounds become more soluble in water and this induces the formation of emulsions. Emulsions make it difficult to effectively separate between solvent layers. Emulsions can be removed through either centrifugation or increasing the polarity of the aqueous layer. Addition of, for example sodium chloride salt, will decrease the solubility of organic compounds in water.
Extraction Process
[edit | edit source]

First, the caffeine from tea leaves can be extracted through solid-liquid extraction. This can be achieved by boiling the tea bag in water and a weak base, such as Calcium Carbonate, in order to break the ester bonds in the tannins. The liquid caffeine can then be transferred out and vacuum filtered. The filtered caffeine can be transferred to a centrifuge tube and is ready for a second extraction with the organic solvent. When the organic solvent is added, the two layers should be allowed to separate before removing the desired organic layer. If emulsions form, centrifuge and add salt accordingly. The liquid caffeine should be extracted multiple times with the organic solvent. The collected organic extracts can be evaporated in a sand bath on a hot plate in order to obtain a "crude" product. This product can be further purified due to the ability of caffeine to sublime. Sublimation is the ability for a compound to go from the solid state to the gas state without having gone through the liquid state. Any impurities will not sublime with the caffeine.[3] The impurities can not sublime with caffeine because they require difference temperatures and pressure. In essence, the caffeine is sublimated from the crude product, allowing experimenters to collect caffeine and leave behind impurities.[4]
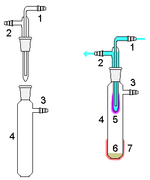
- Sublimation:
Sublimators can be set-up in a multitude of ways but the main goal is to heat the solid inside a sublimation chamber and have it reach the gas state and collect on a tube or apparatus inserted into the sublimation chamber. After the sublimation is complete, the tube can be removed and the collected caffeine can be taken off.[3] The tube is attached to a vacuum which lowers the overall pressure in the apparatus. This lowering of the pressure insures that the caffeine sublimates instead of melt then vaporize. Also the lowering of the pressure does not allow the impurities to sublimate, allowing the experimenter to collect pure caffeine crystals.[4]
MVIE Method of Extraction
[edit | edit source]Microwave Vacuum Ice Water Extraction (MVIE) is a method in which caffeine is extracted by using the power of microwaves. It has been tested with green tea leaves and resulted in a high percent removal yield. This process is dependent on the solvent to solid ratio, microwave power, and time of extraction. With proper execution of technique and time, it extracts approximately 87.6% of caffeine. MVIE proves to be a more effective method to extract caffeine than hot water extractions.
- Basic Procedure
A mixture of tea leaves and water undergo a microwave extraction process then a vacuum ice water extraction. The samples are then placed into a vacuum oven where vapors are collected in a separate container. Multiple extractions are taken to extract a sufficient amount of caffeine from the leaves.
- Factors
Microwave power: With an increase from 160W to 350W, the amount of caffeine extracted was found to be 22% and 46.6% respectively. This indicates that an increase in microwave power increases the amount of caffeine extracted. An increase in power increases internal heat, which ultimately speeds up the process of releasing solvents from the leaves into the solution. However anything higher than 350W will result in the opposite – a decrease in caffeine extraction.
Solvent to solid ratio: the ratio that produces the highest yield of extraction is 10:1 (ml of solvent: g of solid)
MVIE introduces a new and effective way to extract caffeine from tea leaves.
References
[edit | edit source]Mohrig, Jerry R., Christina Noring Hammond, and Paul F. Schatz. Techniques in Organic Chemistry. New York: W. H. Freeman and Company, 2010. Print.
P. Nawrot, S. Jordan, J. Eastwood, J. Rotstein, A. Hugenholtz & M. Feeley (2003): Effects of caffeine on human health, Food Additives and Contaminants, 20:1, 1-30
Winston, Anthony P. "Neuropsychiatric Effects of Caffeine." Advances in Psychiatric Treatment 11 (2005): 432–39. Web.
Lou Z., Er C., Li J., Wang H., Zhu S., Sun J. (2011): Removal of caffeine from green tea by microwave-enhanced vacuum ice water extraction, Anal Chim Acta, 49-53
Images: WikiMedia Commons
- ↑ a b Ashihara, H, & Ashihara, H. (2004). Distribution and biosynthesis of caffeine in plants. Frontiers in bioscience, 9(1-3), 1864-.
- ↑ a b Mohanpuria, P, & Mohanpuria, P. (2009). Caffeine biosynthesis and degradation in tea [Camellia sinensis (L.) O. Kuntze] is under developmental and seasonal regulation. Molecular biotechnology, 43(2), 104-.
- ↑ a b Mohrig, Jerry R., Christina Noring Hammond, and Paul F. Schatz. Techniques in Organic Chemistry. New York: W. H. Freeman and Company, 2010. Print.
- ↑ a b extraction, October 28, 2012


