Structural Biochemistry/Carbohydrates/Aldoses
General information
[edit | edit source]An Aldose contains an aldehyde with two or more hydroxyl groups attached; one of the hydroxyl groups is at end opposite to the aldehyde. An Aldose is a type of monosaccharides, which is a chiral molecule that plays a key role in the development of nucleic acids. The two simplest forms of Aldoses are L- and D-Glyceraldehydes, which are three-carbon structures that each contain one aldehyde and two hydroxyl groups. The L and D symbols apply to the two different configurations of the asymmetric carbon farthest from the aldehyde group.

In the figure below, the common D-aldose sugars are shown:

Family Tree of D-Aldoses.jpg
Hemiacetal
[edit | edit source]The carbonyl group in aldehydes and ketones may react with one molecule of alcohol to form a hemiacetal. The ‘-OR’ group in alcohol attacks the carbonyl carbon in aldehydes or ketones, thus breaking the ‘C-O’ double bond. Proton transfer either intramolecularly or via solvent completes the reaction. Hemiacetal formation may be either acid or base catalyzed. Under acidic condition, however, the carbonyl group may react one more time when alcohol is in excess to form an acetal. The following two diagrams depict an example of hemiacetal and acetal found in carbohydrates.
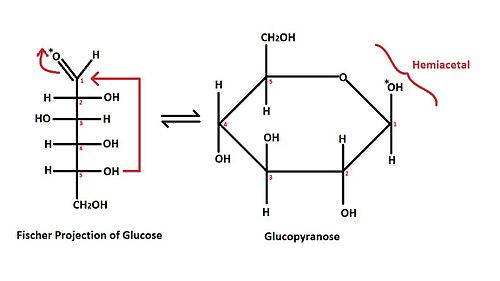

The red arrows shown in the Fischer projection of a glucose molecule demonstrate the brief overview of mechanism for the hemiacetal formation. The (*) mark on the oxygen of aldehyde indicates the position of the oxygen after the ring formation, while the numbers on each stereocenters indicate the position of each carbon.

Ring formation
[edit | edit source]Most of the sugars form cyclic rings, which are more stable than the open chain form. To enable ring formation, the aldehyde can react with an alcohol to form a hemiacetal. Carbohydrates may form either five or six membered rings depending on which hydroxyl group undergoes the hemiacetal formation. A five membered ring is called furanose, while a six membered ring is called pyranose. Furanoses form when the hydroxyl group on C4 reacts with the carbonyl group, while pyranoses form when the hydroxyl group on C5 reacts. This forms the intramolecular hemiacetal in the ring structure. In carbohydrates, the hemiacetal/acetal carbon (C1) in cyclic form is called the anomer. This carbon may be labeled as α or β depending on the position of the (*)-labeled oxygen in the figure. If the (*)-labeled oxygen in the picture is above the ring, the anomeric carbon is labeled as β. If below, it is labeled as α. The following is the formation of a five membered ring by a glucose molecule:

