Structural Biochemistry/Chemical Bonding
Introduction
[edit | edit source]Atoms form bonds by gaining, losing, or sharing electrons. Typically, they seek to achieve the electron configuration of a noble gas. Bonding occurs because it lowers the energy of a system and makes the atom more stable. Atoms also a unit of matter, the smallest unit of an element, consisting of a dense, central, positively charged nucleus surrounded by a system of electrons and an equal in number to the number of nuclear protons. The entire structure having an approximate diameter of 10-8 centimeter and characteristically remaining undivided in chemical reactions except for limited removal, transfer, or exchange of certain electrons.
An atom cannot be broken down further without changing the chemical nature of the substance. For example, if you have 1 ton, 1 gram or 1 atom of oxygen, all of these units have the same properties. We can break down the atom of oxygen into smaller particles, however, when we do the atom looses its chemical properties. For example, if you have 100 watches, or one watch, they all behave like watches and tell time. You can dismantle one of the watches: take the back off, take the batteries out, peer inside and pull things out. However, now the watch is no longer behaves like a watch.
Atoms are made up of 3 types of particles: electrons, protons and neutrons. Each of these particle has different properties. Electrons are tiny, very light particles that have a negative electrical charge (-). Protons are much larger and heavier than electrons and have the opposite charge. So, protons have a positive charge (+). Neutrons are large and heavy like protons, however, neutrons have no electrical charge. Anyway, each atom is made up of a combination of these particles. The proton and electron stay together just like the two magnets (the opposite electrical charges attract each other).
The electron that is constantly spinning around the center of the atom (called the nucleus). The centrigugal force of the spinning electron keeps the two particles from coming into contact with each other much as the earth's rotation keeps it from plunging into the sun. In an electrically neutral atom, the positively charged protons are always balanced by an equal number of negatively charged electrons. As we have seen, hydrogen is the simplest atom with only one proton and one electron. Helium is the 2nd simplest atom. It has two protons in its nucleus and two electrons spinning around the nucleus. With helium though, we have to introduce another particle. Because the two protons in the nucleus have the same charge on them, they would tend to repel each other, and the nucleus would fall apart. To keep the nucleus from pushing apart, helium has two neutrons in its nucleus. Neutrons have no electrical charge on them and they act as a sort of nuclear glue, holding the protons, and thus the nucleus together.
As you add more electrons, protons and neutrons, then the size of the atom increases. We can measure an atom's size in two ways: 1) By using the atomic number (Z) or using the atomic mass (A, also known as the mass number). The atomic number describes the number of protons in an atom. For hydrogen, the atomic number (Z) is equal to 1. For helium Z = 2. Since the number of protons equals the number of electrons in the neutral atom, Z also tells you the number of electrons in the atom. The atomic mass tells you the number of protons plus neutrons in an atom. Therefore, the atomic mass, A, of hydrogen is 1. For helium A = 4.
Electrically neutral atoms are the atoms that have no positive or negative charge on them. Atoms, however, can have electrical charges. Some atoms can either gain or lose electrons (the number of protons never changes in an atom). If an atom gains electrons, then the atom becomes negatively charged. If the atom loses electrons, then the atom becomes positively charged (because the number of positively charged protons will exceed the number of electrons). An atom that carries an electrical charge is called an ion.
While the number of protons for a given atom never changes, the number of neutrons can change. Two atoms with different numbers of neutrons are called isotopes. For example, an isotope of hydrogen exists in which the atom contains 1 neutron (commonly called deuterium). Since the atomic mass is the number of protons plus neutrons, two isotopes of an element will have different atomic masses (however the atomic number, Z, will remain the same).
The quantum mechanical basis for the formation of chemical bonds is an overlap in the probability densities of two or more wavefunctions; this means that electrons have a non-zero chance of being found in a region of space that is forbidden by classical physics.
Ionic Bonds
[edit | edit source]
An ionic bond is the transfer of electrons between a metal and a nonmetal. An example of an ionic bond is that between sodium and chlorine atoms. The sodium atom transfers its lone electron in the 3s state to the chlorine atom. After the electron transfer, the sodium atom bears a +1 charge while the chlorine atom now bears a -1 charge. With this transfer of electrons, the sodium now has the electron configuration of the noble gas neon, while chlorine now has that of the noble gas argon.
Ionic bonding will occur only if the overall energy change for the reaction is favorable – when the bonded atoms have a lower energy than the free ones. The larger the resulting energy change the stronger the bond. The low electronegativity of metals and high electronegativity of non-metals means that the energy change of the reaction is most favorable when metals lose electrons and non-metals gain electrons.
Pure ionic bonding is not known to exist. All ionic compounds have a degree of covalent bonding, which means, ionic bond could be consider as a special type of covalent bond. The larger the difference in electronegativity between two atoms, the more ionic the bond. Ionic compounds conduct electricity when molten or in solution. They generally have a high melting point and tend to be soluble in water.
Covalent Bonds
[edit | edit source]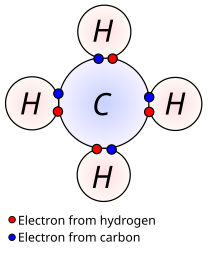
Covalent bonds are another type of chemical bond used to achieve a noble gas configuration, or an octet of electrons. Covalent bonds are formed between nonmetals, usually from the Boron, Carbon, Nitrogen, Oxygen, and Halogen families. Metals are rarely involved in covalent bonds. Each covalent bond consists of two electrons, one usually from each atom involved in the bond. The atoms form enough covalent bonds that when the electrons in the bonds are added with the valence electrons, they will have an octet. The key difference between ionic and covalent bonds lies in how the electrons are distributed between the two atoms. In ionic bonds, the electrons are transferred from one atom to the other, giving the atoms effective +1 and -1 charges. However, in covalent bonds, the valence electrons from both of the two atoms are shared between two atoms. Thus, neither atom is given a full positive or negative charge. Instead, the electrons shared between the two atoms - whether it be 2, 4, or 6 electrons - varies from molecule to molecule.
There are two types of covalent bonds: pure covalent bonds and polar covalent bonds. Pure covalent bonds exist when there is no difference between the two atoms sharing the electrons. The electronegativity of the two atoms is identical. Because the electronegativity values do not differ, they pull the electrons that are being shared between them with the same force. Thus, the electrons are shared equally and none of the atoms bears a partial positive or negative charge. An example of a pure covalent bond is a Cl-Cl or a Br-Br bond. Pure covalent bonds rarely exist for bonds that are not between identical atoms. Another example would be the covalent bonds between the carbons in long alkane chains.
Polar covalent bonds are those that exist between atoms of different electronegativities. The electrons in the bond are still being shared, but not equally between the two atoms. Though the exact ratio of the electron density that each atom bears cannot be determined easily, it is very easy to determine which atom pulls more electron density towards itself. The more electronegative atom will pull the shared electrons more, causing it to now bear a slightly negative charge. Because charge has to be conserved, the less electronegative atom must now bear a slight positive charge, equal in magnitude to the negative charge. As an example, consider a bond between carbon and chlorine. Chlorine is much more electronegative than carbon, thus it pulls more of the electrons towards itself. This gives the chlorine a slightly negative charge and the carbon a slightly positive charge. If the difference between the two atoms is so great causing one of the two atoms to posess a lot of the electron density, the bond becomes increasingly ionic and less covalent. For this reason, though H-Cl is considered a covalent bond, it is classified as a very strong acid, meaning it dissociates completely. Because the electronegativity difference is so vast, the chlorine molecule pulls all the electron density towards itself, thereby dissociating into H+ and Cl- ions in the presence in water.
However, it is important to note that a molecule that contains polar bonds can be nonpolar. For example, take the molecule carbon tetrachloride. This molecule has four polar C-Cl bonds. However, due to the orientation of the polar bonds, they cancel out and the molecule as a whole is nonpolar.
Hydrogen Bonds
[edit | edit source]
A hydrogen bond is a bond created by the dipole-dipole interaction of a hydrogen atom and an electronegative atom such as an oxygen or nitrogen atom due to dipole dipole interactions. A common example of this is water where the electronegativity of the oxygen allows it to have a slight negative charge while the two hydrogen atoms have a slight positive charge. The negative charge on the oxygen forms a weak bond with the slight positive charge of another water molecule's hydrogen. This type of bonding is also present in organic fluorine compounds between C and F groups. This force is weaker than covalent bond and ionic bonds, but stronger than Van der Waals interactions.
Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C), or most of the solutions that use water as the solvent. This is because of the strong hydrogen bond, as opposed to other group 16 hydrides. Intramolecular hydrogen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids.
Role of Noncovalent Interactions in Macromolecules
[edit | edit source]In macromolecules such as proteins, DNA, and RNA, noncovalent interactions are essential. Noncovalent interactions include hydrogen, ionic, hydrophobic, and Van Der Waals bonding. These interactions are described in more specificity in the list that follows this group. When compared to covalent bonds, noncovalent bonds are weak and continuously form and break bonds. However, when several noncovalent bonds are formed, there is a net increase in bond strength. Their combined participation in a macromolecule makes a difference (i.e. substrate binding to enzyme and the lipid bilayer's role in transport). With several hydrogen bonds, ionic, and hydrophobic interactions existent at the same time, it is unlikely that these several weak interactions will break the substrate and enzyme without external energy. This property is the reason why enzymes have specific catalytic power. Protein folding and the unique properties and structures of proteins also depend on these noncovalent interactions.
Metallic Bonding
[edit | edit source]Metallic bonding is the bonding between metal and metal. The bonding involves electron pooling and metallic bonding. Since metal atoms are larger and can easily lose their valence electrons, the electrons on their outer shell can be pooled and be distributed evenly with other metal atoms. Differently from the covalent bonding, electrons in the metallic bonding are delocalized which means that the electrons can move freely through the metal.

1) Metallic bonding in sodium: Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Even a metal like sodium (melting point 97.8°C) melts at a considerably higher temperature than the element (neon) which precedes it in the periodic table. Sodium has the electronic structure 1s22s22p63s1. When sodium atoms come together, the electron in the 3s atomic orbital of one sodium atom shares space with the corresponding electron on a neighbouring atom to form a molecular orbital. This is the same sort of way that a covalent bond is formed. The difference, however, is that each sodium atom is being touched by eight other sodium atoms, the sharing occurs between the central atom and the 3s orbitals on all of the eight other atoms. And each of these eight is in turn being touched by eight sodium atoms, which in turn are touched by eight atoms and so on, until you have taken in all the atoms in that lump of sodium. All of the 3s orbitals on all of the atoms overlap to give a vast number of molecular orbitals, which extend over the whole piece of metal. There have to be huge numbers of molecular orbitals, because each orbital can only hold two electrons. The electrons can move freely within these molecular orbitals. Each electron becomes detached from its parent atom. The electrons are said to be delocalized. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons.
2) Metallic bonding in magnesium: Magnesium has the outer electronic structure 3s2. Both of these electrons become delocalized, so the "sea" has twice the electron density as it does in sodium. The remaining ions also have twice the charge and so there will be more attraction between ions and sea. Each magnesium atom has 12 protons in the nucleus compared with sodium's 11. In both cases, the nucleus is screened from the delocalized electrons by the same number of inner electrons,the 10 electrons in the 1s2 2s2 2p6 orbitals. That means that there will be a net pull from the magnesium nucleus of 2+, but only 1+ from the sodium nucleus. So not only will there be a greater number of delocalized electrons in magnesium, but there will also be a greater attraction for them from the magnesium nuclei. Magnesium atoms also have a slightly smaller radius than sodium atoms, and so the delocalized electrons are closer to the nuclei. Each magnesium atom also has twelve near neighbors rather than sodium's eight. Both of these factors increase the strength of the bond.
3)Metallic bonding in transition elements: Transition metals tend to have particularly high melting points and boiling points. The reason is because they can involve in the 3d electrons in the delocalization as well as the 4s. The more electrons can involve, the stronger the attraction.
4) The metallic bond in molten metals: In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. The metallic bond is not fully broken until the metal boils. That means that boiling point is actually a better guide for the strength of the metallic bond than melting point. In melting, the bond is loosened and is not broken.
Relative Strength of Chemical Bonds and Intermolecular Forces
[edit | edit source]All the bonds known to chemistry can be described the various dissociation energies needed to break the bonds. It is these dissociation energies that rank the strength of the various bonds found in chemistry. Ionic bonds, being the strongest bonds, have a dissociation energy of >400 kcal/mol. Covalent bonds where are the second strongest bonds have a dissociation energy of about 400 kcal/mol. Hydrogen bonds, dipole-dipole, and london (van der waals) dispersion bonds are in a sub category of bonds labeled intermolecular forces. These forces are significantly weaker than ionic and covalent bonds because of their nature of being interactive forces between compounds rather than physical bonds. Hydrogen bonds are the strongest of said forces and overall third strongest in all bond interactions with a dissociation energy of 12-16 kcal/mol. Dipole Dipole interactions are the second strongest intermolecular force but the fourth strongest bond interaction with a dissociation energy of 0.5-2 kcal/mol. Lastly comes the london vander waal forces that the weakest interaction in chemical bonding with a dissociation energy of <1 kcal/mol.
References
[edit | edit source]Berg, Jeremy M. Biochemistry. 6th ed. W.H. Freeman, 2007.
- ↑ Silberberg, Martin S.(2010). Principles of General Chemistry (2nd Edition).McGraw Hill Publishing Company. ISBN978-0-07-351108-05
