Structural Biochemistry/Chemistry of important organic molecules in Biochemistry/Vitamin C
Vitamin C
[edit | edit source]Vitamin C (ascorbic acid), is an essential nutrient for humans and certain animals. It is a cofactor in many enzymatic activities and it prevents the disease scurvy. It can also act as an antioxidant against oxidative stress.
Vitamin C was first named as “ascorbic acid” by Walter Norman Haworth after it was discovered to be an anti-scurvy factor. “Ascorbic” means “against scurvy”. Casimir Funk introduced the term “vitamin C” around 1920.
Biochemical Role
[edit | edit source]An accumulation of mutations in the gene coding for I-guolonolactone oxidase (enzyme in ascorbic biosynthetic pathway) caused primates and a few other animal species unable to synthesize ascorbic. One theory as to why this happened is that maybe the loss of being able to synthesize ascorbic promoted mutations to occur for more genetic variability and therefore helping to further evolution. Another theory is that the vitamin C in fruits and vegetables was sufficient enough to compensate for not being able to synthesize it. A byproduct of synthesizing ascorbic is hydrogen peroxide so the loss may have been advantageous.
Scurvy is caused by ascorbic deficiency. The symptoms include vision problems, neurological disorders and the alteration of the extracellular matrix in blood vessels, bones, skin, gums, and tendons. Many of the symptoms can be explained by the inactivation of a few ascorbic-dependent enzymes. These enzymes commonly share a catalytic mechanism that requires Fe2+, 2-oxoglutarate, and ascorbic as co-substrates. The enzymes are in the class of 2-oxoglutarate dependent dioxygenases (2-ODDs).
Different 2-ODDs catalyze reactions such as hydroxylation, desaturation, and oxidative ring-closure or expansion. These reactions lead to the addition of oxygen into a substrate. These steps are critical in a large number of biochemical pathways. Ascorbic is thought to merely maintain the Fe2+ state but many electron donors do not seem to be able to replace it in maintaining 2-ODDs in their active form. Studies on the mechanism of peptidyl-prolyl-4-hydroxylase (P4H) show the complexity of the reaction. Without ascorbic, P4H is quickly inactivated by self-oxidation. Inactivation of P4H from lack of ascorbic is the first identified cause of scurvy. The hydroxylation of peptidyl-proline is necessary for collagen folding. In humans, unhydroxylated collagen is more flexible and it is degraded in the endoplasmic reticulum (ER). Different collagen types have specific functions in maintaining skin, tendons, cartilage, bones, teeth, cornea, muscles, blood vessels, other tissues, and organs. Collagen is the most abundant protein in animals.
Vitamin C, also called ascorbic acid, is much more than just an antioxidant that prevents scurvy. It plays a very important role in signaling that occurs in cells as well as gene expression. In addition to 2-ODDs helping incorporate oxygen into organic substrates, ascorbic acid also participates in hypoxia-inducible factor (HIF) hydroxylation signaling. This reaction requires the presence of oxygen because it is catalyzed by dioxygenases [1]. Hypoxic (low oxygen) conditions are obviously not favored for this reaction, so animal cells have developed a fascinating way to detect if oxygen is present and actually have defenses available against hypoxia. HIF1α is a transcription factor, which contains two proline residues that are hydroxylated. This is the basis behind oxygen detection in mammalian cells [3, 4]
The HIF family contains transcriptionally activating genes, HIF1 is specifically involved in angiogenesis, metabolism, nutrient transport, and cell migration [5]. HIF1 is a heterodimer that contains both an α and β subunits. Two proline residues of HIF1α (Pro402 and Pro564) are hydroxylated by prolyl hydroxylases (HIF-P4H) [1]. The HIF-P4H complex is located in the cytosol and its Km value for oxygen are above atmospheric concentration. This shows that the HIF-P4H complexes are able to successfully sense oxygen levels.
Normoxic conditions (when there is oxygen available) allow the proline residues of HIF1α to be hydroxylated. This is a necessary step for the von-Hippel-Lindau (pVHL) protein to bind. Subsequently, HIF1α undergoes degradation by a proteasome-mediated pathway. Hypoxia conditions prevent hydroxylation from taking place, and thus HIF1α is unable to bind to pVHL. So, HIF1α cannot be degraded and it is transported to the nucleus. Here, HIF1α binds with HIF1β, forming a dimer that binds the Hypoxia Responsive Element in the promoter region of a vast variety of hypoxia-induced genes. These genes play important roles in cancer such as angiogenesis [6]. Ascorbic acid may have potential to help in certain cancer treatments. This is a highly debated idea but is centered around the concept that HIF1α has the ability to stimulate angiogenesis, which plays a major role in cancer growth [6]. It has also been shown that cancer patients can have lower amounts of ascorbic acid [7].
HIF1α builds up in hypoxic conditions as well as from nonhypoxic stimulation. Nonhypoxic stimuli, can come from pyruvate and oxaloacetate from the Krebs cycle. The nonhypoxic activation of HIF is partially controlled by ascorbic acid [1].
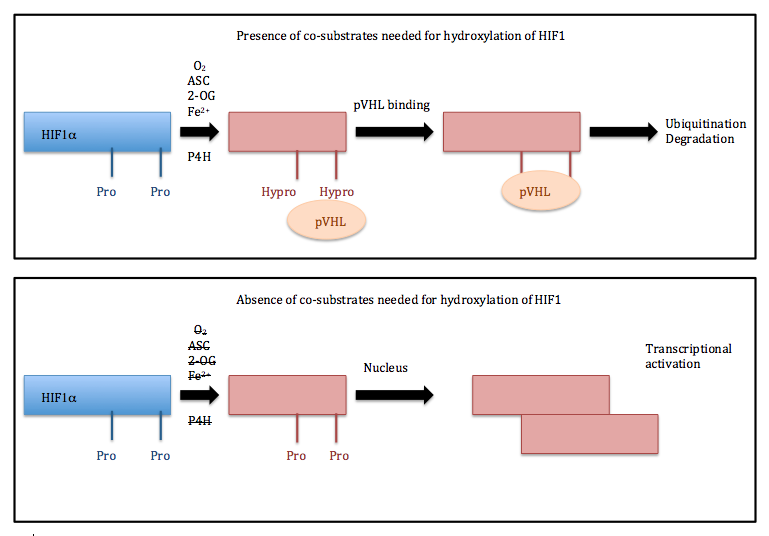 Different pathways for HIF1 depending on presence of co-substrates needed for its hydroxylation.
Different pathways for HIF1 depending on presence of co-substrates needed for its hydroxylation.
Gene Expression
[edit | edit source]Ascorbic is thought to be involved in gene transcription, RNA stabilization, post-translational modification of proteins, hydroxylation, epoxidation and desaturation of many substrates. This hypothesis suggests that ascorbic acid seems to stabilize certain mRNAs. It was observed that stabilization of collagen transcript is ascorbic dependent. Ascorbic is required for the expression of osteocalcin which is a calcium-binding protein that is made by osteoblasts. The mechanisms of how ascorbic controls gene expression is still unclear. Ascorbic acid has also been observed to induce cell differentiation. It is hoped that ASC can be used for in vitro synthesis of differentiated cells from embryonic stem cells. This can be useful in future clinical treatment.
Antioxidant
[edit | edit source]Ascorbic acid is a well-known antioxidant. It acts as a reducing agent and reverses oxidation in liquids. Oxidative stress is caused by there being more free radicals in the body than there are antioxidants. This condition affects cardiovascular disease, hypertension, diabetes, and chronic inflammatory diseases.
Non-antioxidant
[edit | edit source]Ascorbic acid is not only an antioxidant, it also behaves as a “non-antioxidant” (Pro-oxidant). It has been shown to reduce transition metals such as iron from Fe3+ to Fe2+.
Vitamin C sources
[edit | edit source]Vitamin C can be found in fruits and vegetables. It is also present in certain meats such as liver. Citrus fruits are the most commonly known sources of vitamin C. The Kakadu plum (Australia) and the camu camu fruit (Amazon Rainforest) have the highest concentration of vitamin C.
References
[edit | edit source]- "Beyond the Antioxidant: The Double Life of Vitamin C - Springer." Home - Springer. N.p., n.d. Web. 6 Dec. 2012. <http://link.springer.com/chapter/10.1007/978-94-007-2199-9_4/fulltext.html>.
- "Vitamin C - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 6 Dec. 2012. <http://en.wikipedia.org/wiki/Vitamin_C>.
[3] Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIF targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science.
[4] Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIFalpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science.
[5] Chun YS, Kim MS, Park JW (2002) Oxygen-dependent and -independent regulation of HIF1α.J Kor Med Sci.
[6] Harris AL (2002) Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer.
[7] Mayland CR, Bennett MI, Allan K (2005) Vitamin C deficiency in cancer patients. Palliat Med.
