Structural Biochemistry/Enzyme/Coenzymes
Coenzymes are one group of cofactors that can either be tightly or loosely bound to the enzyme. The former are called prosthetic groups, whereas the latter are like cosubstrates. Coenzymes are small organic molecules and are often derived from vitamins making them crucial components in biological reactions. Enzymes that use the same coenzyme perform similar catalysis mechanisms.
Common Coenzymes
[edit | edit source]NADH
[edit | edit source]NADH, Dinucleutide a naturally occurring coenzyme found in all living cells, triggers energy production and helps supply cells with energy. NADH dehydrogenase is an enzyme specifically placed in the mitochondrial membrane. NADH catalyzes the transfer of electrons from NADH to coenzyme Q (CoQ). It plays vital roles in the mitochondrial electron transport chain being the first enzyme (complex I). NADH + H+ + CoQ + 4H+in → NAD+ + CoQH2 + 4H+out
Through this reaction the complex 4 protons are translocated across the inner membrane per molecule of oxidized NADH, contributing to the production of ATP through the electrochemical potential that was established. The reaction is as well reversible is there a high presence of membrane potential.
Composition and structure
NADH dehydrogenase contains 45 separate polypeptide chains, making it the largest of the respiratory complexes. Essential components that are of functional importance are the eight iron-sulfur clusters and the flavin prosthetic group. The mitochondrial genome encodes seven of the 45 subunits. NADH possess the structure of an “L” shape with a long membrane domain and a hydrophilic peripheral domain, which accommodates all the known redox centres and the NADH binding site.
Structure:
FADH
[edit | edit source]Flavin adenine dinucleotide FAD: flavin adenine dinucleotide acts as a redox cofactor associated with important reactions that engage metabolism. FAD changes between two redox state accounting for its’ biochemical role. Derived from riboflavin also known as vitamin B2 consists of a riboflavin group that is bound to the phosphate group of an adenosine diphosphate. FAD accepts two hydrogen atoms to be reduced into the FADH. FADH is associated to be an energy-carrying molecule, and can also be incorporated in the mitochondria as a substrate to attain the oxidative phosphorylation process. The structure of FAD is shown below:
Quinone
[edit | edit source]The basic structure of quinones consists of any member of a class of cyclic organic compounds that containing two carbonyl groups, C=O, either adjacent or separated by a vinylene group, −CH = CH−, in a six-membered unsaturated ring. Quinones are an important chemical structure as it relates to color in biological organisms. For example, quinones are present in biological pigments such as biochromes. Some include benzoquinones, naphthoquinones, anthraquinones, and polycyclic quinones. The quinones are found in bacteria, fungi, various higher plant forms, and are sometimes found in animals. An example of a quinone is coenzyme Q, also known as ubiquinone. Ubiquinone is hydrophobic and diffuses rapidly in inner mitochondrial membranes; its structure is shown below:
CoA
[edit | edit source]Coenzyme A or CoA is derived from pantothenic acid and adenosine triphosphate (ATP) and used in metabolism in areas such as fatty acid oxidization and the citric acid cycle. Its main function is to carry acyl groups such as acetyl as thioesters. A molecule of coenzyme A carrying an acetyl group is also referred to as acetyl-CoA. Coenzymes are sometimes denoted CoA, CoASH, or HSCoA. One form of Coenzyme A is Acetyl-CoA. Acetyl-CoA is a very important because it is a precursor to HMG CoA. Acetyl-CoA is involved in cholesterol and ketone synthesis. And is vital component to the acetyl group in acetylcholine. Acetyl coenzyme A is a key component in the krebs cycle where pyruvate is converted to acetyl CoA. This coenzyme has a sulfur atom which bonds to the acetyl fragment by an unstable bond which makes it very reactive, the enzyme is now ready to feed its acetate into the krebs cycle for further oxidation. Since coenzyme A is chemically a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier. It assists in transferring fatty acids from the cytoplasm to mitochondria. A molecule of coenzyme A carrying an acetyl group is also referred to as acetyl-CoA. When it is not attached to an acyl group it is usually referred to as 'CoASH' or 'HSCoA'.
The structure of CoA-SH is shown below:
Thiamine Pyrophosphate
[edit | edit source]Thiamine pyrophosphate (TPP) is a thiamine (vitamin B1) derivative produced by the enzyme thiamine pyrophosphotase. As a coenzyme, it is present in all living systems and is important for catalyzing several biochemical reactions. It was first discovered while studying the peripheral nervous system disease Beriberi, which results from a deficiency of thiamine in the diet. Research has shown that TPP is an essential nutrient in humans, capable of preventing such a disease. TPP is a prosthetic group in many enzymes, such as: Pyruvate dehydrogenase complex, Pyruvate decarboxylase complex in ethanol fermentation, Alpha-ketoglutarate dehydrogenase complex, Branched-chain amino acid dehydrogenase complex, 2-hydroxyphytanoyl-CoA lyase, and Transketolase.
Chemical Structure
[edit | edit source]
TPP consists of a pyrimidine ring that is connected to a thiazole ring, which is in turn connected to a pyrophosphate (diphosphate) functional group. The thiazole ring component is the most chemically involved part of TPP in reactions, since in contains reactive nitrogen and sulfur parts. This component is considered the “reagent portion” of the molecule. The C2 carbon of this ring participates in some reactions by acting as an acid and donating its proton to form a carbanion. This negatively charged carbanion is stabilized by the positive charge on the adjacent tetravalent nitrogen, making the reaction more favorable. This type of compound is known as the “ylid form”.
Pyridoxal phosphate
[edit | edit source]
Also known as PLP or pyridoxal-5’-phosphate (P5P), it is a prosthetic group of some enzymes. It is the active form of vitamin B6, which comprises three natural organic compounds, pyridoxal, pyridoxamine, and pyridoxine. As a coenzyme, it is involved in transamination reactions and in some decarboxylation and deanimation reactions of amino acids. Its aldehyde goup forms a Schiff-base linkage with the ε-amino group of a specific lysine group of the aminotransferase enzyme. Afterwards, the ε-amino group of the active-site lysine residue is displaced by the α-amino group of the amino acid substrate. This results in a quinoid intermediate from a deprotonated external aldimine, which in turn can accept a proton at a different position to become a ketamine. in turn accepts a proton at a different position to become a ketimine. This ketamine is hydrolyzed so that the amino group remains on the complex.
PLP is also involved in the synthesis of the neurotransmitters serotonin and norepinephrine and of heme (a molecular constituent of hemoglobin) and in the conversion of the amino acid tryptophan to the vitamin niacin.
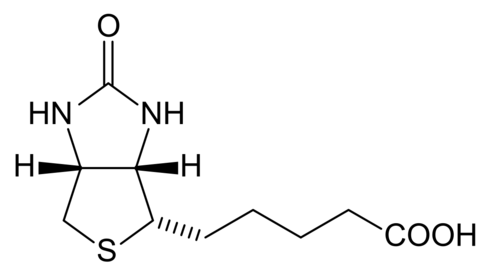
Biotin
[edit | edit source]Biotin is which also known as vitamin H, vitamin B7, or coenzyme R that is tightly bounded to an enzyme (prosthetic group). Biotin is essential in the formation of fatty acids and glucose. Furthermore, it also aids in the metabolism of carbohydrates, fats, and proteins. It also helps promote healthy hair and skin. Biotin is a water-soluble B –complex vitamin that consists of a tetrahydroimidizalone ring that is fused with a tetrahydrothiophene ring. Biotin contains a valeric acid substituent that is attached to one of the carbons on the tetrahydrothiophene. Biotin works by activating enzymes (pyruvate carboxylase) that are responsible for the rearrangement of glucose, amino acids, and fatty acid molecules. Deficiency of biotin is quite rare. This deficiency is caused by excessive consumption of raw egg whites and can be addressed with supplements.
Tetrahydrofolate
[edit | edit source]Tetrahydrofolate, also known as tetrahydrofolic acid is a derivative of folic acid. It is a coenzyme that is essential in the metabolism of amino acids and nucleic acids. Furthermore, it is crucial to interconvert amino acids, methylate tRNA, and generate formate. It is produced from dihydrofolic acid by dihydrofolate reductase in the liver. It acts as a donor group involved in the transfer of single carbon groups. Tetrahydrofolate is transported across cells by receptor-mediated endocytosis. Tetrahydrofolate is used to treat megaloblastic and macrocytic anemias which results from a deficiency in folic acid.

5'-Deoxyadenosyl Cobalamin
[edit | edit source]5-deoxyadenosyl cobalamin is one of the two forms of vitamin B12 that is used in the body. 5-deoxyadenosyl cobalamin is a coenzyme that is needed by the enzyme methylmalonyl mutase that converts L-methylmalonyl-CoA to succinyl-CoA. The conversion is an essential step in extracting energy from fats and proteins in the body. Also, the production of succinyl-CoA is crucial for the production of hemoglobin, the oxygen binding protein that carries oxygen from the lungs to tissues.
Uridine diphosphate N-acetylglucosamine
[edit | edit source]Uridine diphosphate N-acetylglucosamine (UDP-GlcNAc)is a nucleotide sugar that acts as a coenzyme in metabolism. It has a role in transferring N-acetylglucosamine residues to substrates by interacting with glycosyltransferases. It is produced in the hexosamine biosynthesis pathway, which initially starts with the synthesis of glucosamine-6-phosphate from fructose 6-phosphate and glutamine. As an end-product of this pathway, it is further utilized in the production of glycosaminoglycans, proteoglycans, and glycolipids.

