Structural Biochemistry/Membrane Proteins/Assembly of Membrane Proteins into Complexes
Membrane Proteins' Role in Complexes
[edit | edit source]Proteins embedded within membranes (integral proteins) perform many crucial functions within the cell, and data has shown that membrane proteins function in complexes. Researchers have been working to identify the factors with which these membrane proteins interact with. Only a limited amount of membrane proteins are compatible with the current methods used to study membrane protein function in complexes, and very limited knowledge on this subject is confidently reported. Scientists are still working in attempts to define the composition of membrane protein complexes.
However, even in light of these difficulties, there are a few methods in existence that have helped researchers to make some headway in terms of analyzing certain membrane protein complexes. In particular, the membrane protein complexes of mitochondria, microsomes, bacteria, and chloroplasts have been examined via blue native-polyacrylamide gel electrophoresis (BN-PAGE). Furthermore, the split-ubitquitin method was utilized to analyze yeast membrane protein interactions as well.
Membrane Protein Complex Assembly
[edit | edit source]In addition to determining the composition of membrane protein complexes, it is also important to understand the vitality of the assembly process in these complexes. Assembly is an ordered process, which is crucial to the overall function of the complex. Larger protein complexes require more intermediates, and it is much more difficult to identify the nature of the assembly intermediates in these cases. In the simpler complexes, scientists have been able to determine the process of assembly order and the intermediates involved. These specific assembly orders play important roles in determining the specific pathway needed to carry out a certain cell function. With a different assembly order, the pathway would possibly lead to a "dead-end" in the systematic process.
A good example of one of these "simpler complexes" that have undergone study is the cytochrome bo3 complex of Escherichia coli. This complex's linear assembly pathway was determined from discovered how the complex is made up of four integral subunit proteins, and assembled by two intermediate complexes. E.coli also proves to be a showing example of the vitality of order in "ordered assembly". For the cell division process within this complex to function normally, 12 proteins are sequentially imported to division site, and they must be ordered in the correct way otherwise correct cell division would not occur, and potentially harmful intermediate complexes can arise from incorrect pathways produced from mistakes in assembly order.
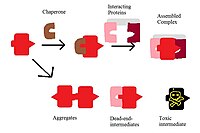
Chaperones
[edit | edit source]A very crucial factor in the function of membrane protein complexes are the chaperones that coordinate their assembly. Such chaperones can interact with the assembled protein complexes and guide them to their ultimate functional goal by preventing unwanted interactions. Unwanted membrane protein complex interactions could then lead to potentially harmful, and non-functional complexes. These unwanted proteins are shown in the figure to the left. Accordingly, without a chaperone, the protein could aggregate, stop at a dead-end intermediate, or become highly toxic. An example of chaperoning is the assembly of F1 in F1F0-ATP synthase. This is a yeast enzyme that is composed of an alpha and beta subunit. The assembly factors Atp12p and Atp11p prevents the assembly from becoming aggregated by binding to the hydrophobic surfaces of the subunits and moving them into an alpha 3 beta 3 complex. The formation of F0 also uses a chaperone called UncI. The UncI chaperones the assembly of the c subunits, which form a homo-oligomeric c-ring. The c subunits would still be capable of assembling with other parts of the F0 machinery in the absence of the UncI chaperone; however without the UncI, it cannot form the c-ring and the complex formed would be an ultimately nonfunction, 'dead-end' intermediate.
Furthermore, chaperones also mediate the process responsible for regulating complex formation. In some cases when there are various proteins and interfaces involved in the assembly process of the complex, it is carried out in a selective manner to which chaperones contribute. Certain interactions may occur at later stages of complex formation, not during intermediate stages as seen previously; chaperones play a role in this regulation. In the study of cbb3-type cytochrome c oxidase in Rhodabacter capsulatas, researchers found that complex assembly occurred through an inactive late assembly intermediate (made up of three structural subunits and two assembly factors). At the end of the process, the complex was only activated when the final structural subunit took the place of the assembly factors previously in place. It is thought that these inactive intermediates may be vital during complex assembly in which incomplete complex assembly could produce harmful consequences, such as in the respiration or photosynthesis complex assembly.
Dynamic Exchange
[edit | edit source]Recent research has also been put into studying dynamic exchange in assembled proteins. This dynamic exchange allows such membrane proteins to easily transfer in and out of the complex they are a part of. Dynamic exchange provides a means of repair: if part of the complex is damaged, instead of rendering the entire complex useless or nonfunctional, that specific protein can be substituted for a functional one. According to this research, membrane proteins are not stable once they are formed. Early research suggested that the proteins in photosystem II in chloroplasts are repaired by exchanges in a new D1 subunit for a photo-damaged D1. Another example of dynamic exchange involves the Translocase of the mitochondrial outer membrane complex. Such experimentation has shown that proteins are carried solely into the mitochondria, and thus they do not pick up any other required subunits needed for complete complex synthesis. Although this brings up a new and exciting fact to protein assembly, it has only been directly proven in vitro or in test tubes and not in vivo. However, three recent research have used fluorescent microscopy on cells containing GFP tagged proteins showing dynamic exchange in vivo.

