Structural Biochemistry/Organic Chemistry/Lipids
Lipids Overview
[edit | edit source]Lipids are naturally occurring (organic) compounds that are insoluble in polar solvents such as water . Their insolubility can be attributed solely to their long hydrophobic hydrocarbon chains. These hydrophobic chains may be saturated or unsaturated. Unsaturated chains contain double or triple covalent bonds between adjacent carbons while saturated chains consist of all single bonds. Lipids are composed of a glycerol molecule bonded to long hydrocarbon chain(s) (can be single or multiple) and, depending on the lipid, to other molecules—such as a phosphate group (phospholipids).
Some examples of the types of lipids are: neutral, saturated, (poly/mono) unsaturated fats and oils (monoglycerides, diglycerides, triglycerides), phospholipids, sterols (steroid alcohols), zoosterols (cholesterol), waxes, and fat-soluble vitamins (vitamins A, D, E, and K). Lipids have many different biological functions such as fuel molecules, structural building blocks for phospholipids and glycolipids, covalent attachments to guide molecules to specific membrane locations, and intracellular messengers.


There are three common types of Membrane Lipids. They are phospholipids, glycolipids, and cholesterol. [Structural Biochemistry].
Fats Fats consists of glycerol and 3 fatty acids. Fats are created via 3 condensation reactions creating ester linkages that link the fatty acid carboxyl groups to the hydroxyl groups in glycerol. There are two different types of fatty acids, saturated and unsaturated. In a saturated fatty acid, it has the maximum number of hydrogen atoms possible, thus there are no double bonds. There are only single bonds. Since saturated fatty acids are only single bonds, it can pack more tightly together at room temperature and this makes it a solid at room temperature. An example of a saturated fatty acid is butter. An unsaturated fatty acid has one more double bonds. These double bonds create a kink in the hydrocarbon tail, which in return results in looser packing. At room temperature, it is a liquid. An example of this is oil.
Phospholipids They are found in biological membranes. The components of phospholipids include a hydrophobic tail and hydrophilic head. The hydrophobic tail consists of two hydrocarbon chains. The hydrophilic head consists of choline, phosphate, and glycerol. The fatty acids give a hydrophobic barrier, whereas the remainder of the molecule has hydrophilic properties. Phospholipids spontaneously form lipid bilayers due to amphipathic nature of lipid molecules. Phospholipids are found in all cell membranes.
Cholesterol Cholesterol is a steroid and they are built from 4 fused hydrocarbon rings. The hydrocarbon tail is connected to the steroid at one end, and a hydroxyl group is connected to the other end. Cholesterol is a steroid important in cell membranes and acts as a precursor to some sex hormones. However, prokaryotes do not have cholesterol.
Triglycerides
[edit | edit source]Neutral fats (triglycerides) are composed of fatty acid hydrocarbon chains bonded to a single glycerol molecule. Fatty acids consist of long hydrocarbon chains with a carboxyl group while glycerol consists of 3 carbons and 3 hydroxyl groups. Fatty acids are the building blocks of fat molecules. The method by which the three fatty acid chains in a triglyceride attach to a single glycerol molecule is called dehydration synthesis. Dehydration synthesis is also used in various other reactions, including the joining of two monosaccharides to form a disaccharide. Triglycerides function primarily in energy storage, as a form of insulation, and to protect and cushion cells and organs.
There is an image of a triglyceride molecule with three neutral fatty acid chains and a glycerol group
Saturated fatty acids contain single bonds between the carbons of the hydrophobic chain. Saturated fatty acids originate from animals and are found as component chains in a triglyceride molecule. Saturated fatty acids exist in the solid state at room temperature. Unsaturated fatty acids however contain one (monounsaturated) or more (polyunsaturated) double bond(s) between the carbons of the hydrocarbon chain, which causes the molecule to bend. Triglycerides with too many bends cannot be packed as closely together as neutral fatty acids and therefore are less dense. Below is an example of a saturated fatty acid

Triglycerides composed of many fatty acids that melt at lower temperatures than those triglycerides with saturated fatty acids. These unsaturated fatty acids do not bind at their maximum number of hydrogen’s because of double bonding between the carbons of the chain. Unsaturated fatty acids originate from plants and are found as component chains to triglyceride molecules. Unsaturated fatty acids exist in the liquid state at room temperature.

These images depict a saturated fatty acid chain (contain single carbon bonds) and an unsaturated fatty acid chain (contain double carbon bonds).
Phospholipids
[edit | edit source]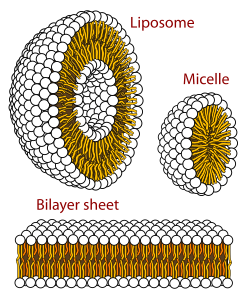
Phospholipids are modified triglycerides with one of the fatty acid chains replaced with a phosphate group. They are made by four distinguished groups: fatty acid chains, a platform, a phosphate group, and an alcohol attached to the phosphate. The fatty acid chains are hydrocarbon chains that are typically 14-24 carbons in length. The platform is either glycerol or sphingosine, which is an amino alcohol with a hydrocarbon chain. Phospholipids have a very characteristic non-polar fatty acid chain portion and a polar phosphate portion.The amphipathic character of phospholipids contribute in its' crucial role in phospholipid bilayers. The polar phosphate group is capable of interacting with water molecules and spontaneously forms a bilayer in an aquatic environment. Phospholipids orientate themselves so that the polar heads are facing the water molecules and the hydrophobic fatty acids are oriented toward the inside of the bi-layer. The bi-layer environment enables the non-polar fatty acid chains to stay together, avoiding the water while the hydrophilic phosphate group is oriented toward the water. Phospholipids participate in the formation of the cell membrane by the coming together of two layers of phospholipids. The phospholipids are responsible for the membrane's semi-permeability and fluidity.
The structure describe a phospholipid:

This image illustrates the components and orientation of the hydrophilic phosphate group and the hydrophobic fatty acid chains that form the lipid bi-layer.
Phospholipid is the most common group of lipids. In fact, cell membranes as well as organized cellular compartments are all made up of these phospholipids. They can form structures called micelles, in which when phosopholipids congregate, the hydrophobic fatty acid tails join together in the center of the sphere away from the aqueous environment and the polar heads are exposed to the outside. Structures such as liposomes can also be artificially formed from these lipids: using high frequency sound waves to sonicate the sample containing phospholipids and molecules of interest to create phospholipid vesicles that contain the molecules of interest. This is often used to deliver drugs to the cells and study how drugs pass through the membrane.
The plasma membrane is made up of the phospholipid bilayer. The membrane is an amphipathic sheet-like structure that is fluid and electrically polarized. The membrane itself has little functions, but the proteins that are integrally and peripherally integrated to the membrane help mediate many of the functions that we contribute to membrane. The membrane is asymmetric in that the proteins are randomly distributed across the membrane, some are attached inside the cell, some outside, and others integrated within membrane. Also, rapid lateral diffusion and slow transverse diffusion contribute both to the membrane's asymmetric characteristic and fluidity. In transverse diffusion, phospholipids are flipped inside-out or outside-in, and this flipping is regulated by flipases. However, the longer the fatty acid chains are, the less likely for transverse diffusion to occur. Longer chains also decreases the fluidity of the plasma membrane. There are other factors that may affect plasma membrane's fluidity. For example, the better arranged the fatty acids chains are, the less fluid the membrane is. On top of that, the more unsaturated the fatty acids are, the more fluid the membrane is. This is because the double bonds bend the chains that allows sloppy arrangements. The interruption of cholesterol within the membrane also causes more fluidity since the polar hydroxy group in cholesterol disrupts the hydrophobic environment within the phospholipid bilayer.
Glycolipids
[edit | edit source]Glycolipids are sugar(glyco-)containing lipids. They are derived from sphingosine instead of a form of phospholipids that derives from glycerol (phospholipids exist in both derivatives from glycerol and sphingomyelin platform). Another difference from phospholipids is that glycolipids contain a sugar unit (can be glucose or galactose) instead of a phosphate group.
Examples: Glycolipid molecules exist from the most basic molecule, cerebroside which contains 1 fatty acid unit, a sphingosine backbone, and 1 sugar unit (glucose or galactose), to the most complex molecules containing branched chains of multiple sugar residues (up to seven residues in gangliosides).
Properties: When glycolipids exist in membranes, their sugar residue terminal always face the extracellular side.
Chemical structure of Glycolipids

Cholesterol
[edit | edit source]
Cholesterol is a form of lipids that differs from the rest of its relatives. It is relatively medium molecule that contains 4 adjacent cyclic hydrocarbon molecules with three six-member rings and one five-member ring that has a hydroxyl and a saturated hydrocarbon chain terminals.
The molecule functions as a bufferor a temperature stabilizer for the membrane in which it can make up of 25% of the membrane. When exist in membranes, the 4 cyclic molecules in the cholesterol molecule lay parallel to the fatty acid chains of the phospholipids, meanwhile the hydroxyl terminal points in the direction with the polar phospholipid heads in which it interact with.
Cholesterol molecules exist primarily in nerve cells. The molecule binds to the myelin sheath membrane which provides an outer coating that protects the nerve cell from its surroundings.
It is an essential predecessor to sex hormones that exists in males (testosterone) and females (oestradiol). Also an essential component in vitamin D that enables the body to utilize calcium to form bones.
Animals acquire very little cholesterol from the food they eat; they make cholesterol within the body. Although cholesterol is essential for many processes and structural function, it can be detrimental to have excess cholesterol. Too much cholesterol in the blood will cause blockages in the arteries which can result in heart disease, high blood pressure, and stroke. Only 0.25% of human beings retain High Cholesterol disease from heredity, however people are gaining high cholesterol in their blood from the food they eat (especially people in America).
Membrane Properties
[edit | edit source]The cell membrane has a set of properties that are contributed to the presences of the lipids as well as proteins. 1. Structures are lik sheets. 2. They are formed by lipids, proteins, and carbohydrates. 3. The membrane is amphipathic (contain hydrophobic and hydrophilic regions). 4. Each protein allows for the function of the membrane. 5. Membranes are held on by weak, non-covalent bonds. 6. The structure is asymmetric. 7. It is high in fluidity. 8. The membrane is polarized.
Fluidity
[edit | edit source]
The presence of the lipids in the membrane structure of a cell is vital for the cell especially affecting its fluidity. As addressed in the section below, this is necessary to allow things to flow in and out of the cell. One factor that plays a big role in this is cholesterol as shown below. Another one is the presence of double bonds. The more the double bonds, the greater the amount of kinks or curve in the lipid and therefore more free space.
The length of the lipids also plays a role. Lipids move in two distinct ways. Most commonly they interact in lateral diffusion where they switch places with the lipid to the left or right of them. Other times, they go through transverse diffusion where they flip flop with the ones whose tails they are facing. This is due to the weak, Van der Waals interaction of the lipid molecules. The longer the lipid is, the stronger this interaction is, therefore decreasing the mobility of the lipids. Decrease lipid mobility yields in decreasing the fluidity of the membrane.
Cholesterol and fluidity
[edit | edit source]
Cholesterol is an important factor in membrane permeability, that is, how much can flow through the cell. Cholesterol acts as a 'buffer' to prevent against any extremes. Obviously, the membranes permeability cannot be too fluid as to allow anything inside the cell(i.e. harmful agents),but at the same time, the membranes permeability must be fluid enough as to let out/let in important agents that need to enter the cell.
Cholesterol is a hydrocarbon steroid with one single alcohol group, which leads to its amphiphatic nature.
Generally,
1.AT LOW TEMPERATURES:Cholesterol in a membrane leads to a more fluid membrane.
2.AT HIGH TEMPERATURES:Cholesterol in a membrane leads to a less fluid membrane.
Ether Lipids with Branched Chains
[edit | edit source]
Two major factor that separates Archea(bacteria) from Bacteria is the Archea's cell membranes phospholipid consists of ether linkages and the fatty acid hydrocarbon chains are completely saturated and branched with a methyl group every 5 carbons. These simple structural differences provides Archeabacteria drastic difference from bacteria in terms of their habitat's harsh environments. These 2 factors contribute to the chemical properties that their membrane is more resistant to hydrolysis (ether versus ester linkages) and resistant to oxidation (branched saturated hydrocarbon chains).
Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
[edit | edit source]Phospholipids and glycolipids have amphipathic characteristics which enables them to form a micelle or a "lipid bilayer". Due to the hydrophobic hydrocarbon tail and the hydrophillic polar head group, the lipids arrange in a form where the polar groups face water while the tail is away from water. One formation is the micelle where the lipids arrange themselves in a circle with the head groups making the circumference while the tails are inside. A more favorable formation is the lipid bilayer or the bimolecular sheet. This arrangement has the lipids form a barrier where the polar head groups face the aqueous media and the hydrophobic tail face inside away from water. This type of formation is favorable to cell membranes for it forms a barrier from the extracellular fluid and protects the cytoplasm within the cell. Integral and peripheral proteins may be present in the lipid bilayer to allow certain functions to occur such as transportation of ions or acting as pumps.
References
[edit | edit source]Berg, Jeremy; Tymoczko, John; Stryer, Lubert. Biochemistry, 6th edition. W.H. Freeman and Company. 2007. Berg, Jeremy M., Tymoczko, John, L., Stryer, Lubert. Biochemistry. Seventh Edition.
The Organics of Biochemistry:[1]
"Lipid bilayer." Wikipedia. 7 December 2012. 7 December 2012 <http://en.wikipedia.org/wiki/Lipid_bilayer>.
Viadiu, Hector. "Lipids and Cell Membranes." UCSD, 19 November 2012.
