Structural Biochemistry/Organic Chemistry/Methods of Purification
Recrystallization
[edit | edit source]
Recrystallization is the process of purification that involves dissolution of a solid in a hot solvent, filtration of the heated solution or mixture, crystal formation, and the isolation of the crystalline compound. In order to perform a recrystallization, the solubility of a compound in a hot solvent must be taken advantage of. A saturated solution at a higher temperature normally contains more solute than the same solute/solvent pair at a lower temperature; as a result, the solute precipitates when a warm saturation solution cools. In other words, a solution at a higher temperature will have more dissolved solids and as it cools, the solute will return back to its solid phase, forming a precipitate. Impurities in the solid being recrystallized are usually significantly lower in concentration than the concentration of the substance being purified so as the mixture cools, the impurities remain in solution while the highly concentrated product crystallizes.
Crystal formation of a solute from a solution is a selective process because only solids moving at the right speed and are under the appropriate conditions of concentration and solvent form almost perfect crystalline materials as only molecules of with the right shape fit into the crystal lattice. Recrystallization purifies a compound because dissolution of the impure solid in a suitable hot solvent destroys the crystal lattice of the impure compound and the recrystallization from the cold solvent selectively produces a new, more pure crystal lattice. Slow cooling of the saturated solution promotes formation of pure crystals because the molecules of the impurities that don’t fit too well have time to return to the solution. Crystals that form slowly are larger(not always) and often purer than ones that form quickly because rapid crystal formation traps impurities within the lattice as they are simply surrounded by the crystallizing solute. The most important aspect of recrystallization is the choice of solvent because the solute should have maximum solubility in the hot solvent and minimum solubility in the cold solvent. The relationship of solute and solvent can be best described as “like dissolves like”. This entails that nonionic compounds generally dissolve in water only when they can associate with the water molecules through hydrogen bonding. Hydrocarbons and alkyl halides are virtually insoluble in water whereas carboxylic acids and alcohols are often recrystallize from water solutions. In a miniscale recrystallization experiment several steps are followed to complete the purification process.
- The solid to be recrystallized must first be weighed and then dissolved in an appropriate hot solvent.
- The solid impurities must be filtered using a gravity filtration process.
- The hot recrystallization mixture is then set aside to cool to room temperature.
- After cooling to room temperature, the solution is then cooled even further by placing it in an ice-water bath for 10-15 minutes to allow further recrystallization.
- To collect the crystals and to complete the recrystallization, the crystals must be collected by vacuum filtration.
An easy depiction of the recrystallization process
[edit | edit source]One of the key factors of purification through recrystallization is understanding solubility. In order to proceed with a successful recrystallization process, the mixture must have some important solubility properties. One solvent must be soluble at all temperatures. The next solvent must be soluble at low temperatures. And another solvent must be soluble at high temps, and insoluble at low temps.
Here is a simple illustration which explains how the purification process is conducted.
The blue square is soluble at all temperatures, The orange circle is mostly insoluble at all temperatures, and the green triangle is soluble at high temperatures and insoluble at low temperatures.
- 1. Add heat: If we heat up the mixture containing the square, circle, and triangle, the square will already be in solution, and we can heat it to the point that the green triangle dissolves. Then we are left with only the insoluble circle.
- 2. Filter: We can then filter the aqueous dissolved solution and once we separate this we are left with only the triangle and square in solution while the circle was left in the previous vessel.
- 3. Cool Down: When the newly isolated mixture is cooled, the triangle will begin to crystallize again since it is insoluble at low temperatures, but the square will remain in solution since it is soluble at all temperatures.
- 4. We separate the aqueous layer(the triangle) and we are left with our compound of interest, the triangle.
Isoelectric Focusing
[edit | edit source]
Isoelectric Focusing is an electrophoresis protein purification process in which proteins are separated by their isoelectric points. The isoelectric point of a protein, the pI, is the characteristic pH at which a protein carries no net charge. The net charge of a protein is determined by the acidity or basicity of the side chains that make up the protein. If a protein has more acidic groups than basic groups then the protein will have a very low pH and be considered acidic. If the protein has more basic groups in its side chains than acidic groups then the overall charge of the protein will cause its pH to be much higher. Isoelectric focusing takes advantage of these properties with the following steps:
- Create a gel that has a linear pH gradient within it
- Insert the protein samples into the gel
- Apply an electric field with an anode (+) at one end and a cathode (-) at the other
- Allow time for the proteins to migrate toward their neutral pI according to their net charge
The linear pH of the gel allows for the samples to be added arbitrarily because the pH of the environment combined with the electric field will force the movement of the protein regardless of their initial positions within the gel. The proteins do not begin to migrate until the electric field is applied. Upon application of the electric field, the proteins move toward the terminal with the charge opposite them gathering or releasing protons throughout migration until they have reached their neutral, isoelectric point. For example, assume all the protein samples have been applied at the neutral pH location of the gradient where pH=7. When the field has finally been applied the proteins will migrate according to their net charge. If the protein has an isoelectric point of 2 then at the pH of 7, the protein has less hydrogen ions than it needs in order to be neutral, i.e. reach its isoelectric point. This deems the protein as negative and thus it will move toward the anode (+) picking up protons as it travels through the gradient to a lower pH. When the protein has finally gathered enough protons to make it neutral, the protein will no longer have a net charge and cease its migration at its isoelectric point.
Isoelectric Focusing allows for protein purification based on a different protein characteristic, pI. Therefore proteins with similar characteristics such a molecular weight can be purified and separated via their distinct pI's in rather short periods of time.[1]
Sublimation
[edit | edit source]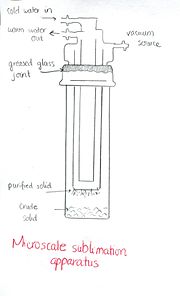
Sublimation is the process in which a substance changes directly from a solid to the gas phase without forming an intermediate liquid phase. One example of this is dry ice, which is converted from the solid form of carbon dioxide directly into carbon dioxide gas. In the laboratory, sublimation can be used to purify an organic compound only after meeting four requirements.
- The compound must vaporize without melting
- It must be stable enough to vaporize without decomposing
- The vapors of the compound must be able to condense back to the solid
- Impurities within the compound do not also sublime.
The apparatus for sublimation consists of an outer vessel and an inner vessel. The outer vessel holds the sample to be purified connected to a vacuum. The inner container known as a cold finger provides a cold surface on which the vaporized compound can recondense as a solid. To perform sublimation, the sample to be sublimed must be placed into a filter flask. Next, an inner tube is placed in the flask and the vacuum is turned on. Afterwards, the sublimation flask is heated gently, using a sand bath, as ice is filled in the inner tube. During sublimation, material will disappear from the bottom of the outer vessel and then reappear on the cool, outside surface of the inner test tube. This is the result of the compound vaporizing as it reaches its sublimation temperature and then recondensing on the cold finger as a result of cooling. After completion, the inner test tube can be removed and the pure solid can be scraped off and analyzed.
It is important to note the distance between the impure compound and the cold finger during sublimation. The components of the sublimation set up need to be close enough to avoid decomposition yet far enough that contamination doesn’t take place. A large distance means that the temperature applied must be very high to keep the compound in the vapor form, which could cause the compound to decompose. A small distance could cause the impurities to easily come into contact with the purified compound on the cold finger. The purification could be ineffective if the distance is not taken into consideration. [2]
Distillation
[edit | edit source]Distillation is used to separate a two-component mixture and Gas Chromatography is used to identify the constituent compounds. Distillation is a technique that separates compounds based on their vapor pressures and boiling points. When two different compounds are heated, one may boil at a lower temperature than the other. By separating the vapors of the compound with the lower boiling point from the other, one may separate the vapor from the liquid and re-condense it, effectively separating the two. With successive distillations, a high degree of purification is possible. Distillations carried out one a time are called simple distillations. Although high efficiency can be achieved through multiple simple distillations, it would be tedious and require a large initial sample volume. Fractionating distillation, however, simplifies this repeated distillation by providing continuous separations. A fractionating column is utilized to provide extensive surface area to allow for heat exchange between rising vapor and falling condensate; through a recursive mechanism, the upper vapors are more pure in the more volatile compound and the liquid is more pure in the less volatile compound. The degree of separation depends on the different boiling points of compounds as well as the rate of distillation, insulation, and column efficiency. By allowing more time to distill, thermal equilibrium can be reached and higher purities achieved. Likewise, insulation prevents heat loss to preserve initial conditions and column efficiency determines how many distillation “pockets” can occur.
Thin Layer Chromatography (TLC)
[edit | edit source]Thin Layer Chromatography (TLC) is a simple and quick procedure for separating and identifying components in a mixture. In principle, different components in a mixture have different solubility and differ in their strength of attraction to an adsorbent. This method utilizes this principle and has the mixture to be analyzed performed on a plate with thin layer of a solid adsorbent and then has the plate immersed in a solvent. Components in the mixture will slowly travel up the plate at different rates until they reach the maximum separation for this particular combination of solvent and adsorbent. After the mixture is separated into different colored spots, the plate is dried and the components are examined.
Technique:
1. Choose a solvent to be used to analyze the mixture, and then pour it into a beaker to a depth less than 0.5cm. The entire process is carried out in a beaker with watch glass on the top to prevent solvent vapor from escaping.
2.A TLC plate is prepared. A TLC plate is made with a thin layer of adsorbent, usually silica or alumina. Near the bottom of the plate, use a pencil to draw a line across the plate. This line will be the origin where you spot the mixture to be analyzed.
3. Place the plate in beaker so that only the bottom of the plate is immersed in the solvent.
4. When the solvent rises up by capillary action and past the spot applied, some components of the mixture will travel at faster rate due to their solubility with the solvent and their adsorption strength to the plate.
5. Different separation of colored spots will be seen on the plate. If the spots are not colored, UV lamp is used to visualize the plate.
To identify the compounds present, the distance travelled by the solvent and the distance travelled by individual spots are then measured from the plate. Using these measurements, a retention factor, Rf is obtained by the following equation:
Rf = (Distance traveled by the compound)/(Distance traveled by the solvent)
From the value of Rf, the polarity of a compound can be predicted. Moreover, this value can be used to compare two compounds. If two substances have the same Rf value, there is a high chance that they are the same compound. Otherwise, they are certainly different compounds.
References
[edit | edit source]- ↑ Andrews, A.T. (1986). Electrophoresis: theory, techniques, and biochemical and clinical applications (2nd edn). Oxford University Press, Oxford.
- ↑ Mohrig, Jerry R. "Techniques in Organic Chemistry." 2010, W.H. Freeman and Company
Mohrig, Jerry R. Techniques in Organic Chemistry. 2006, W.H. Freeman and Company
Organic Chemistry Lab, Chemistry 143A

