Structural Biochemistry/Organic Chemistry/Organic Functional Group/Carbonyl/Ketone

A ketone is another functional group under the general groups of carbonyls. In a ketone, the carbonyl carbon is doubly-bonded to an oxygen, and single bonded to two alkyl groups, which can be either identical or different. It is important for both “R” groups to be alkyl groups – if the “R” group is an “H”, it becomes an aldehyde, a carboxylic acid if the “R” group is an “OH”, and an ester if the “R” group is “OR.”
The carbon atom next to the carbonyl carbon is the alpha-carbon. By analogy, the hydrogen’s attached to the alpha-carbon are the alpha-hydrogens.
Nomenclature
[edit | edit source]
IUPAC Nomenclature
1. In terms of functional group priority, ketones take priority over everything but carboxylic groups and aldehydes.If an aldehyde or carboxylic acid group is there, the ketone is no longer the highest priority and thus, becomes an oxo- prefix.
2. First count the longest carbon chain which contains the carbonyl compound. Name this chain according to normal IUPAC rules.
3. Number the chain such that the carbonyl carbon has the lowest number.
4. The oxygen is designated in the name with the number of the carbon it is on.
5. The suffix of ketones is "-one" so the "e" in the alkyl chain is replaced with "-one."
Another method of naming ketones exists though this method is not used as much. In this method of nomenclature, the two alkyl groups on either side of the carbonyl group are mentioned separately. For example, look at the example of propan-2-one or 2-propanone. Another way of naming this, would be to take the carbons #2 and #3 as one group and #1 as another. The carbonyl carbon becomes a part of the longer alkyl chain - in this example, both are equivalent so it does not matter. Then each side is named and the word "ketone" put at the end of it. Thus, 2-propanone could also be referred to as ethyl methyl ketone.
Common Names
Like other carbonyl compounds, ketones have common names that are used more often in everyday jargon. An example is acetone. The chemical formular of acetone is C3H6O. The IUPAC name is propanone but is often referred to as acetone.
Reactivity
[edit | edit source]Like the other carbonyl groups, the carbonyl carbon has a large positive charge due to the very electronegative oxygen atom. This characteristic of the ketones allows it to undergo numerous reactions as detailed below:
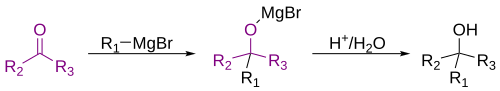
1. Nucleophilic addition – nucleophilic addition is very common for ketones. Because of the polarity of the carbonyl carbon, nucleophilic attack is a typical reaction ketones undergo. Thus, the carbonylcarbon is subject to nucleophilic attack from other excellent nucleophiles such as cyanide and hydroxide ions. The lone pair of electrons on the nucleophile attacks the positively charged carbon, which moves one of the two bonds shared between the carbon and oxygen onto the oxygen, in the process giving oxygen a full negative charge. For example, a reaction of a ketone and water results in geminal diols.
2. Grignard – Ketones can be completely transformed, resulting in another functional group. For example, grignard reagents or organometallic reagants will result in a tertiary alcohol. Of course, this is after an aqueous workup is used – aqueous work up in this case is a solution of acid and water. When using grignard reagants it is important to remember that a carbon-carbon bond is formed.