Structural Biochemistry/Personalized Medicine
Overview
[edit | edit source]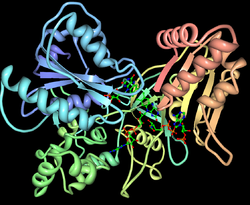

Despite the many pharmacological advancements achieved in the past few decades through structural biochemistry, prescribed medications work in fewer than fifty percent of the patients who take them. The reason for this is that, while mostly similar, everyone’s genome is slightly different and responds differently to the same medications. The underlying cause is that there exist variants in the genes that make Cytochrome P450. Cytochrome P450, an example of which is pictured at right, refers to a large and diverse family of enzymes that process the drugs that we take. Therefore, there are as many different responses to the drugs that we take as there are variants in the gene.
If one knows a patients entire genome, however, it would be relatively easy to predict the types of medications that would work and which ones would be least effective. This is the idea behind personalized medicine, a medical model that uses information from a patient’s genome and proteome to optimize his or her medical care. Personalized medicine is the ultimate goal on the medicinal stairway model, pictured at right. The lowest step is the use of blockbuster drugs; the more advanced step is the stratified medicine level; and the top step is personalized medicine, the most specific and accurate of the three techniques to patient care.
In the previous fifty years, the primary medical model has been that of the “blockbuster drugs,” or medicines that work for a majority of the generic population. Specifically, a blockbuster drug refers to a drug that generates more than $1 billion of revenue for the patent owner each year. Some examples of past blockbuster drugs are Lipitor, Celebrex, and Nexium. Leading scientists in the field of biochemistry acknowledge that we are slowly leaving the blockbuster drug era and are in the midst of moving to the second level, stratified medicine.
Stratified medicine refers to managing a patient group that has shared biological characteristics, such as the presence or absence of a gene mutation. Molecular diagnostic testing is used to confirm these similarities; then, the most optimal treatment is selected in hopes of achieving the best possible result for the group. An example of stratified medicine in practice is grouping patients with breast cancer who have estrogen receptor positivity or HER2 over-expression, and who can be treated according to these characteristics with an anti estrogens or a HER2 inhibitor.
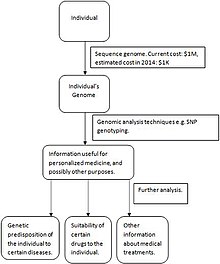
Lastly, personalized medicine is the desired goal we have yet to fully reach. Proteomic profiling, metabolomic analysis, and genetic testing of each individual are required to optimize preventative and therapeutic care. The diagram pictured on the right summarizes the steps needed for personalized medicine to be successful. First, an individual’s genome is sequenced. As technological sequencing techniques advance, the cost of sequencing a genome will decrease, making the personalized medicine model more accessible and more economical. Then, genomic analysis techniques such as SNP genotyping and microarrays are used to gather information about which medicines will work best (for example, regarding an individual’s genetic predisposition toward certain diseases and how long a certain drug will be effective). A current example of the progress made toward personalized medicine is the measurement of erbB2 and EGFR proteins in breast, lung and colorectal cancer patients are taken before selecting proper treatments. As the personalized medicine field advances, molecular information elucidated from tissues will be combined with a patient’s medical and family history, data from imaging, and a multitude of laboratory tests to develop more effective treatments for a wider variety of conditions.
Because everyone has a unique set of genome, advantages of having personalized medicine through pharmacogenetic approaches include:
1. Increase effectiveness of the drug For example, using the right medicine and dosage in order to allow it absorb more easily by a patient’s body
2. Minimize side effects
References
[edit | edit source]PricewaterhouseCoopers’ Health Research Institute,(2009). [The new science of personalized medicine] http://www.pwc.com/personalizedmedicine
Shastry BS (2006). "Pharmacogenetics and the concept of individualized medicine". Pharmacogenomics J. 6 (1): 16–21.
Pharmaceutical Market Trends, 2010-2014, from Urch Publishing
Jørgensen JT, Winther H. The New Era of Personalized Medicine: 10 years later. Per Med 2009; 6: 423-428.
