User:Thomas.haslwanter/Collections
While the human brain may make us what we are, our sensory systems are our windows and doors to the world. In fact they are our ONLY windows and doors to the world. So when one of these systems fails, the corresponding part of our world is no longer accessible to us. Recent advances in engineering have made it possible to replace sensory systems by mechanical and electrical sensors, and to couple those sensors electronically to our nervous system. While to many this may sound futuristic and maybe even a bit scary, it can work magically. For the auditory system, so called “cochlea implants” have given thousands of patients who were completely deaf their hearing back, so that they can interact and communicate freely again with their family and friends. Many research groups are also exploring different approaches to retinal implants, in order to restore vision to the blind. And in 2010 the first patient has been implanted with a “vestibular implant”, to alleviate defects in his balance system.
The wikibook “Sensory Systems” wants to present our sensory systems from an engineering and information processing point of view. On the one hand, this provides some insight in the sometimes spectacular ingenuity and performance of our senses. On the other hand, it provides some understanding of how our senses transduce external information into signals that our central nervous system can work with, and how – and how well - this process can be replaced by technical components.
Introduction
[edit | edit source]The main function of the balance system, or vestibular system, is to sense head movements, especially involuntary ones, and counter them with reflexive eye movements and postural adjustments that keep the visual world stable and keep us from falling. An excellent, more extensive article on the vestibular system is available on Scholorpedia [1]. An extensive review of our current knowledge about the vestibular system can be found in "The Vestibular System: a Sixth Sense" by J Goldberg et al [2].
Anatomy of the Vestibular System
[edit | edit source]Labyrinth
[edit | edit source]Together with the cochlea, the vestibular system is carried by a system of tubes called the membranous labyrinth. These tubes are lodged within the cavities of the bony labyrinth located in the inner ear. A fluid called perilymph fills the space between the bone and the membranous labyrinth, while another one called endolymph fills the inside of the tubes spanned by the membranous labyrinth. These fluids have a unique ionic composition suited to their function in regulating the electrochemical potential of hair cells, which are as we will later see the transducers of the vestibular system. The electric potential of endolymph is of about 80 mV more positive than perilymph.
Since our movements consist of a combination of linear translations and rotations, the vestibular system is composed of two main parts: The otolith organs, which sense linear accelerations and thereby also give us information about the head’s position relative to gravity, and the semicircular canals, which sense angular accelerations.
| Human bony labyrinth (Computed tomography 3D) | Internal structure of the human labyrinth |
|---|---|
 |

|
Otoliths
[edit | edit source]The otolith organs of both ears are located in two membranous sacs called the utricle and the saccule which primary sense horizontal and vertical accelerations, respectively. Each utricle has about 30'000 hair cells, and each saccule about 16'000. The otoliths are located at the central part of the labyrinth, also called the vestibule of the ear. Both utricle and saccule have a thickened portion of the membrane called the macula. A gelatinous membrane called the otolthic membrane sits atop the macula, and microscopic stones made of calcium carbonate crystal, the otoliths, are embedded on the surface of this membrane. On the opposite side, hair cells embedded in supporting cells project into this membrane.

Semicircular Canals
[edit | edit source]
Each ear has three semicircular canals. They are half circular, interconnected membranous tubes filled with endolymph and can sense angular accelerations in the three orthogonal planes. The radius of curvature of the human horizontal semicircular canal is 3.2 mm [3].
The canals on each side are approximately orthogonal to each other. The orientation of the on-directions of the canals on the right side are [4]:
| Canal | X | Y | Z |
|---|---|---|---|
| Horizontal | 0.32269 | -0.03837 | -0.94573 |
| Anterior | 0.58930 | 0.78839 | 0.17655 |
| Posterior | 0.69432 | -0.66693 | 0.27042 |
(The axes are oriented such that the positive x-,y-,and z-axis point forward, left, and up, respectively. The horizontal plane is defined by Reid's line, the line connecting the lower rim of the orbita and the center of the external auditory canal. And the directions are such that a rotation about that vector, according to the right-hand-rule, excites the corresponding canal.) The anterior and posterior semicircular canals are approximately vertical, and the horizontal semicircular canals approximately horizontal.
Each canal presents a dilatation at one end, called the ampulla. Each membranous ampulla contains a saddle-shaped ridge of tissue, the crista, which extends across it from side to side. It is covered by neuroepithelium, with hair cells and supporting cells. From this ridge rises a gelatinous structure, the cupula, which extends to the roof of the ampulla immediately above it, dividing the interior of the ampulla into two approximately equal parts.
Haircells
[edit | edit source]The sensors within both the otolith organs and the semicircular canals are the hair cells. They are responsible for the transduction of a mechanical force into an electrical signal and thereby build the interface between the world of accelerations and the brain.

Hair cells have a tuft of stereocilia that project from their apical surface. The thickest and longest stereocilia is the kinocilium. Stereocilia deflection is the mechanism by which all hair cells transduce mechanical forces. Stereocilia within a bundle are linked to one another by protein strands, called tip links, which span from the side of a taller stereocilium to the tip of its shorter neighbor in the array. Under deflection of the bundle, the tip links act as gating springs to open and close mechanically sensitive ion channels. Afferent nerve excitation works basically the following way: when all cilia are deflected toward the kinocilium, the gates open and cations, including potassium ions from the potassium rich endolymph, flow in and the membrane potential of the hair cell becomes more positive (depolarization). The hair cell itself does not fire action potentials. The depolarization activates voltage-sensitive calcium channels at the basolateral aspect of the cell. Calcium ions then flow in and trigger the release of neurotransmitters, mainly glutamate, which in turn diffuse across the narrow space between the hair cell and a nerve terminal, where they then bind to receptors and thus trigger an increase of the action potentials firing rate in the nerve. On the other hand, afferent nerve inhibition is the process induced by the bending of the stereocilia away from the kinocilium (hyperpolarization) and by which the firing rate is decreased. Because the hair cells are chronically leaking calcium, the vestibular afferent nerve fires actively at rest and thereby allows the sensing of both directions (increase and decrease of firing rate). Hair cells are very sensitive and respond extremely quickly to stimuli. The quickness of hair cell response may in part be due to the fact that they must be able to release neurotransmitter reliably in response to a threshold receptor potential of only 100 µV or so.

Regular and Irregular Haircells
[edit | edit source]While afferent haircells in the auditory system are fairly homogeneous, those in the vestibular system can be broadly separated into two groups: "regular units" and "irregular units". Regular haircells have approximately constant interspike intervals, and fire constantly proportional to their displacement. In contrast, the inter-spike interval of irregular haircells is much more variable, and their discharge rate increases with increasing frequency; they can thus act as event detectors at high frequencies. Regular and irregular haircells also differ in their location, morphology and innervation.
Signal Processing
[edit | edit source]Peripheral Signal Transduction
[edit | edit source]Transduction of Linear Acceleration
[edit | edit source]The hair cells of the otolith organs are responsible for the transduction of a mechanical force induced by linear acceleration into an electrical signal. Since this force is the product of gravity plus linear movements of the head,
it is therefore sometimes referred to as gravito-inertial force. The mechanism of transduction works roughly as follows: The otoconia, calcium carbonate crystals in the top layer of the otoconia membrane, have a higher specific density than the surrounding materials. Thus a linear acceleration leads to a displacement of the otoconia layer relative to the connective tissue. The displacement is sensed by the hair cells. The bending of the hairs then polarizes the cell and induces afferent excitation or inhibition.

While each of the three semicircular canals senses only one-dimensional component of rotational acceleration, linear acceleration may produce a complex pattern of inhibition and excitation across the maculae of both the utricle and saccule. The saccule is located on the medial wall of the vestibule of the labyrinth in the spherical recess and has its macula oriented vertically. The utricle is located above the saccule in the elliptical recess of the vestibule, and its macula is oriented roughly horizontally when the head is upright. Within each macula, the kinocilia of the hair cells are oriented in all possible directions.
Therefore, under linear acceleration with the head in the upright position, the saccular macula is sensing acceleration components in the vertical plane, while the utricular macula is encoding acceleration in all directions in the horizontal plane. The otolthic membrane is soft enough that each hair cell is deflected proportional to the local force direction. If denotes the direction of maximum sensitivity or on-direction of the hair cell, and the gravito-inertial force, the stimulation by static accelerations is given by
The direction and magnitude of the total acceleration is then determined from the excitation pattern on the otolith maculae.
Transduction of Angular Acceleration
[edit | edit source]The three semicircular canals are responsible for the sensing of angular accelerations. When the head accelerates in the plane of a semicircular canal, inertia causes the endolymph in the canal to lag behind the motion of the membranous canal. Relative to the canal walls, the endolymph effectively moves in the opposite direction as the head, pushing and distorting the elastic cupula. Hair cells are arrayed beneath the cupula on the surface of the crista and have their stereocilia projecting into the cupula. They are therefore excited or inhibited depending on the direction of the acceleration.

This facilitates the interpretation of canal signals: if the orientation of a semicircular canal is described by the unit vector , the stimulation of the canal is proportional to the projection of the angular velocity onto this canal
The horizontal semicircular canal is responsible for sensing accelerations around a vertical axis, i.e. the neck. The anterior and posterior semicircular canals detect rotations of the head in the sagittal plane, as when nodding, and in the frontal plane, as when cartwheeling.
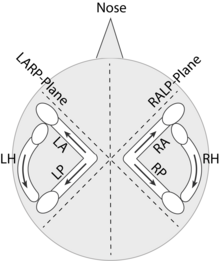
In a given cupula, all the hair cells are oriented in the same direction. The semicircular canals of both sides also work as a push-pull system. For example, because the right and the left horizontal canal cristae are “mirror opposites” of each other, they always have opposing (push-pull principle) responses to horizontal rotations of the head. Rapid rotation of the head toward the left causes depolarization of hair cells in the left horizontal canal's ampulla and increased firing of action potentials in the neurons that innervate the left horizontal canal. That same leftward rotation of the head simultaneously causes a hyperpolarization of the hair cells in the right horizontal canal's ampulla and decreases the rate of firing of action potentials in the neurons that innervate the horizontal canal of the right ear. Because of this mirror configuration, not only the right and left horizontal canals form a push-pull pair but also the right anterior canal with the left posterior canal (RALP), and the left anterior with the right posterior (LARP).
Central Vestibular Pathways
[edit | edit source]The information resulting from the vestibular system is carried to the brain, together with the auditory information from the cochlea, by the vestibulocochlear nerve, which is the eighth of twelve cranial nerves. The cell bodies of the bipolar afferent neurons that innervate the hair cells in the maculae and cristae in the vestibular labyrinth reside near the internal auditory meatus in the vestibular ganglion (also called Scarpa's ganglion, Figure 10.1). The centrally projecting axons from the vestibular ganglion come together with axons projecting from the auditory neurons to form the eighth nerve, which runs through the internal auditory meatus together with the facial nerve. The primary afferent vestibular neurons project to the four vestibular nuclei that constitute the vestibular nuclear complex in the brainstem.

Vestibulo-Ocular Reflex (VOR)
[edit | edit source]An extensively studied example of function of the vestibular system is the vestibulo-ocular reflex (VOR). The function of the VOR is to stabilize the image during rotation of the head. This requires the maintenance of stable eye position during horizontal, vertical and torsional head rotations. When the head rotates with a certain speed and direction, the eyes rotate with the same speed but in the opposite direction. Since head movements are present all the time, the VOR is very important for stabilizing vision.
How does the VOR work? The vestibular system signals how fast the head is rotating and the oculomotor system uses this information to stabilize the eyes in order to keep the visual image motionless on the retina. The vestibular nerves project from the vestibular ganglion to the vestibular nuclear complex, where the vestibular nuclei integrate signals from the vestibular organs with those from the spinal cord, cerebellum, and the visual system. From these nuclei, fibers cross to the contralateral abducens nucleus. There they synapse with two additional pathways. One pathway projects directly to the lateral rectus muscle of eye via the abducens nerve. Another nerve tract projects from the abducens nucleus by the abducens interneurons to the oculomotor nuclei, which contain motor neurons that drive eye muscle activity, specifically activating the medial rectus muscles of the eye through the oculomotor nerve. This short latency connection is sometimes referred to as three-neuron-arc, and allows an eye movement within less than 10 ms after the onset of the head movement.
For example, when the head rotates rightward, the following occurs. The right horizontal canal hair cells depolarize and the left hyperpolarize. The right vestibular afferent activity therefore increases while the left decreases. The vestibulocochlear nerve then carries this information to the brainstem and the right vestibular nuclei activity increases while the left decreases. This makes in turn neurons of the left abducens nucleus and the right oculomotor nucleus fire at higher rate. Those in the left oculomotor nucleus and the right abducens nucleus fire at a lower rate. This results in the fact than the left lateral rectus extraocular muscle and the right medial rectus contract while the left medial rectus and the right lateral rectus relax. Thus, both eyes rotate leftward.
The gain of the VOR is defined as the change in the eye angle divided by the change in the head angle during the head turn
If the gain of the VOR is wrong, that is, different than one, then head movements result in image motion on the retina, resulting in blurred vision. Under such conditions, motor learning adjusts the gain of the VOR to produce more accurate eye motion. Thereby the cerebellum plays an important role in motor learning.
The Cerebellum and the Vestibular System
[edit | edit source]It is known that postural control can be adapted to suit specific behavior. Patient experiments suggest that the cerebellum plays a key role in this form of motor learning. In particular, the role of the cerebellum has been extensively studied in the case of adaptation of vestibulo-ocular control. Indeed, it has been shown that the gain of the vestibulo-ocular reflex adapts to reach the value of one even if damage occur in a part of the VOR pathway or if it is voluntary modified through the use of magnifying lenses. Basically, there are two different hypotheses about how the cerebellum plays a necessary role in this adaptation. The first from (Ito 1972;Ito 1982) claims that the cerebellum itself is the site of learning, while the second from Miles and Lisberger (Miles and Lisberger 1981) claims that the vestibular nuclei are the site of adaptive learning while the cerebellum constructs the signal that drives this adaptation. Note that in addition to direct excitatory input to the vestibular nuclei, the sensory neurons of the vestibular labyrinth also provide input to the Purkinje cells in the flocculo-nodular lobes of the cerebellum via a pathway of mossy and parallel fibers. In turn, the Purkinje cells project an inhibitory influence back onto the vestibular nuclei. Ito argued that the gain of the VOR can be adaptively modulated by altering the relative strength of the direct excitatory and indirect inhibitory pathways. Ito also argued that a message of retinal image slip going through the inferior olivary nucleus carried by the climbing fiber plays the role of an error signal and thereby is the modulating influence of the Purkinje cells. On the other hand, Miles and Lisberger argued that the brainstem neurons targeted by the Purkinje cells are the site of adaptive learning and that the cerebellum constructs the error signal that drives this adaptation.
Alcohol and the Vestibular System
[edit | edit source]As you may or may not know from personal experience, consumption of alcohol can also induce a feeling of rotation. The explanation is quite straightforward, and basically relies on two factors: i) alcohol is lighter than the endolymph; and ii) once it is in the blood, alcohol gets relatively quickly into the cupula, as the cupula has a good blood supply. In contrast, it diffuses only slowly into the endolymph, over a period of a few hours. In combination, this leads to a buoyancy of the cupola soon after you have consumed (too much) alcohol. When you lie on your side, the deflection of the left and right horizontal cupulae add up, and induce a strong feeling of rotation. The proof: just roll on the other side - and the perceived direction of rotation will flip around!
Due to the position of the cupulae, you will experience the strongest effect when you lie on your side. When you lie on your back, the deflection of the left and right cupula compensate each other, and you don't feel any horizontal rotation. This explains why hanging one leg out of the bed slows down the perceived rotation.
The overall effect is minimized in the upright head position - so try to stay up(right) as long as possible during the party!
If you have drunk way too much, the endolymph will contain a significant amount of alcohol the next morning - more so than the cupula. This explains while at that point, a small amount of alcohol (e.g. a small beer) balances the difference, and reduces the feeling of spinning.
- ↑
Kathleen Cullen and Soroush Sadeghi (2008). "Vestibular System". Scholarpedia 3(1):3013.
{{cite web}}: Text "doi:10.4249/scholarpedia.3013" ignored (help) - ↑
JM Goldberg, VJ Wilson, KE Cullen and DE Angelaki (2012). "The Vestibular System: a Sixth Sense"". Oxford University Press, USA.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Curthoys IS and Oman CM (1987). "Dimensions of the horizontal semicircular duct, ampulla and utricle in the human". Acta Otolaryngol. 103: 254–261.
- ↑
Della Santina CC, Potyagaylo V, Migliaccio A, Minor LB, Carey JB (2005). "Orientation of Human Semicircular Canals Measured by Three-Dimensional Multi-planar CT Reconstruction". J Assoc Res Otolaryngol. 6(3): 191–206.
{{cite journal}}: CS1 maint: multiple names: authors list (link)






