High School Chemistry/Electron Configurations of Main Group Elements
It probably seems like all we've been spending a lot of time learning about protons… and neutrons… and electrons… and electrons… and more electrons… so you might be wondering – when do we actually get to study chemistry? When do we get to study reactions? When do we get to study explosions? When do we get to study plastics, and medicines that can be made by combining different kinds of chemicals? The answer is now. We're finally ready to discuss the chemical properties of the simplest chemicals out there – we're finally ready to discuss the elements. Remember, you have learned that there were 118 different kinds of atoms, and that each was known as an element. And you have learned that atoms of different elements have different numbers of protons. Hydrogen has 1 proton (and 1 electron if it's neutral), helium has 2 protons (and 2 electrons, if it's neutral), and lithium has 3 protons (and 3 electrons, if it's neutral). Finally, you have seen examples of the Periodic Table. Scientists use the Periodic Table to summarize information about all of the known elements that exist in our world.
In this lesson, you will learn why the Periodic Table (as shown below) has such an unusual shape.

Now, what's the first thing you thought when you saw the Periodic Table? If you're like most people, the first thing you thought was probably something like, "Wow – that's a funny shape! Why is the Periodic Table shaped like that? Why is it lower in the middle? Why is it higher on either end? Why is there that odd-looking disconnected piece at the bottom? The Periodic Table doesn't look like a table at all!" In this chapter, you'll begin to see why the Periodic Table has such a funny shape. It turns out that the shape of the Periodic Table actually helps to tell us about the chemical properties of the different elements that exist in our world. In this section, for example, you'll learn that elements in the same column of the Periodic Table have similar chemical properties. Later we'll take a look at how elements in the same row are related.
Group 1A (IA) Elements Have One s Electron
[edit | edit source]Remember that according to the Aufbau principle electrons are added to low energy orbitals first and then, as the low energy orbitals are filled up, electrons go into higher and higher energy orbitals. When one atom reacts with another atom in a chemical reaction, it's the high-energy electrons that are involved.
Since it's only the high-energy electrons that participate in a chemical reaction, it's only the high-energy electrons that we will concern us when we want to determine the chemical properties of a particular element. Just how "high" in energy does an electron need to be to participate in a chemical reaction? Well, in most chemical reactions, the only electrons involved are the electrons in the highest energy level. In other words, the electrons with the highest value of n (the principal quantum number), participate in chemical reactions, while the electrons with lower values of n are called "core electrons", are closer to the nucleus and, as a result, don't get involved. The electrons with the highest value of n are known as valence electrons. Core electrons are also referred as non-valence electrons. Two different elements have similar chemical properties when they have the same number of valence electrons in their outermost energy level.
Elements in the same column of the Periodic Table have similar chemical properties. So what does that mean about their valence electrons? You guessed it! Elements in the same column of the Periodic Table have the same number of valence electrons – that's why they have similar chemical properties. Let's see if this is true for some of the elements in the first column of the Periodic Table.
|
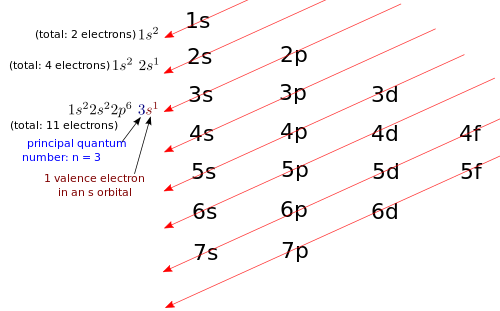
Example 1 – Hydrogen Write the electron configuration for hydrogen (H). Solution: First, you need to find hydrogen on the Periodic Table. Take a look at the Periodic Table above. You know that hydrogen is in the first column, and if you look carefully, you'll see that hydrogen also happens to be at the top of the first column. The Periodic Table tells you that the atomic number for hydrogen is Z = 1, thus hydrogen has 1 proton. Neutral hydrogen will also have 1 electron. You need to write the electron configuration for an atom with 1 electron. As shown in the figure below, the diagonal rule applied to hydrogen (H). Therefore, we write the electron configuration for H: 1s1. Remember, when you write electron configurations, the number out in front always indicates the principal quantum number, n, of a particular orbital, thus 1s2 has n = 1, while 3s1 has n = 3. What is the highest principal quantum number that you see in hydrogen's electron configuration? It's n = 1, so all electrons with n = 1 are valence electrons. Hydrogen has 1 valence electron in an s orbital. |
|
Example 2 – Lithium Write the electron configuration for lithium (Li). Solution: First, you find lithium on the Periodic Table. The Periodic Table tells you that the atomic number for lithium is Z = 3, thus lithium has 3 protons. Neutral lithium will also have 3 electrons. You need to write the electron configuration for an atom with 3 electrons. As illustrated in the figure below, the diagonal rule applied to lithium (Li). Non-valence electrons: 1s2. Therefore, we write the electron configuration for Li: 1s22s1. What is the highest principal quantum number that you see in lithium's electron configuration? It's n = 2, so all electrons with n = 2 are valence electrons, and all electrons with n < 2 are non-valence electrons. Lithium has 1 valence electron in an s orbital. |
|
Example 3 – Sodium Write the electron configuration for sodium (Na). Solution: First, you find sodium on the Periodic Table. The Periodic Table tells you that the atomic number for sodium is Z = 11, thus sodium has 11 protons. Neutral sodium will also have 11 electrons. You need to write the electron configuration for an atom with 11 electrons. As shown below, the diagonal rule applied to sodium (Na). Non-valence electrons: 1s22s22p6. Therefore, we write the electron configuration for Na: 1s22s22p63s1. What is the highest principal quantum number that you see in sodium's electron configuration? It's n = 3, so all electrons with n = 3 are valence electrons, and all electrons with n < 3 are non-valence electrons. (Don't be fooled by the 2p6 orbitals. Even though they are p orbitals, not s orbitals, they have n = 2, so they are non-valence electrons!) Sodium has 1 valence electron in an s orbital. |
If you look at the last line in Example 1, Example 2, and Example 3 you should notice a pattern.
- Hydrogen has 1 valence electron in an s orbital
- Lithium has 1 valence electron in an s orbital
- Sodium has 1 valence electron in an s orbital
In fact, all elements in the first column of the Periodic Table have 1 valence electron in an s orbital. Therefore, we would expect all of these elements to have similar chemical properties – and they do. (Hydrogen is special because it is the first element in the Periodic Table. As a result, hydrogen has only one proton and one electron, which give it special chemical properties. Sometimes scientists don't include hydrogen in the first column of the Periodic Table, but instead give it its own "special" column to reflect its special properties – we won't do that here, but you should realize that hydrogen does not have all the same chemical properties as the rest of the elements in its column.)
The elements in the first column of the Periodic Table (other than hydrogen) are known as Group 1A metals, or alkali metals. When you compare the chemical properties of these elements (lithium, sodium, potassium, rubidium, cesium, and francium), what you'll notice is that they are all remarkably similar. Group 1A elements are metals, silver-colored, and soft. These elements are extremely reactive. Several of them explode if you put them in water.
As pictured below, notice how the elements lithium (Li), sodium (Na), and potassium (K) all look alike. They are all soft, silver metals. Since Li, Na, and K are all Group 1A metals, they all share similar chemical properties.
And finally, because they are so reactive, Group 1A elements are not found in their elemental form in nature – in other words, you don't find pure sodium or pure potassium in nature.
Group 2A (IIA) Elements Have Two s Electrons
[edit | edit source]All of the elements in the first column of the periodic table have 1 valence electron in an s sublevel. How do you think the elements in the second column of the periodic table differ? Let's find out by taking a look at a few examples.
|
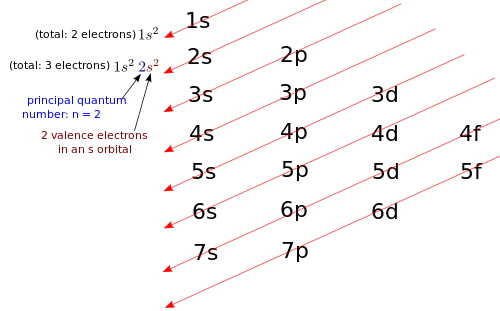
Example 4 – Beryllium Write the electron configuration for beryllium (Be). Solution: First, you find beryllium on the Periodic Table. The Periodic Table tells you that the atomic number for beryllium is Z = 4, thus beryllium has 4 protons. Neutral beryllium will also have 4 electrons. You need to write the electron configuration for an atom with 4 electrons. As shown below, the diagonal rule applied to beryllium (Be). Non-valence electrons: 1s2. Therefore, we write the electron configuration for Be: 1s22s2. What is the highest principal quantum number that you see in beryllium’s electron configuration? It's n = 2, so all electrons with n = 2 are valence electrons, and all electrons with n < 2 are non-valence electrons. Beryllium has 2 valence electrons in an s orbital. |
|
Example 5 – Magnesium Write the electron configuration for magnesium (Mg). Solution: First, you find magnesium on the Periodic Table. The Periodic Table tells you that the atomic number for magnesium is Z = 12, thus magnesium has 12 protons. Neutral magnesium will also have 12 electrons. You need to write the electron configuration for an atom with 12 electrons. Therefore, the electron configuration for Mg: 1s22s22p63s2. What is the highest principal quantum number that you see in magnesium's electron configuration? It's n = 3, so all electrons with n = 3 are valence electrons, and all electrons with n < 3 are non-valence electrons. Magnesium has 2 valence electrons in an s orbital. |
Notice that:
- Beryllium has 2 valence electrons in an s orbital.
- Magnesium has 2 valence electrons in an s orbital.
You can probably guess the number and type of valence electrons in an atom of calcium (Ca), strontium (Sr), barium (Ba), or radium (Ra). If you guessed 2 electrons in an s orbital, then you guessed right! All elements in the second column of the Periodic Table have 2 valence electrons in an s orbital.
The elements in the second column of the Periodic Table are known as Group 2A metals, or alkaline earth metals. As you might expect, because all Group 2A metals have 2 valence electrons in an s orbital, they all share similar chemical properties. Group 2A elements are metals, silver colored, and are quite reactive though they are not nearly as reactive as the Group 1A elements.
Group 3A (IIIA) Elements Have s and 1p Electrons
[edit | edit source]All of the elements in the first column of the Periodic Table have 1 valence electron in an s sublevel and all of the elements in the second column of the Periodic Table have 2 valence electrons in an s sublevel. Can you make any prediction about the valence electrons in the third column of the Periodic Table? Where is the third column of the Periodic Table? It turns out that there are really two different "third columns" in the Periodic Table. Take a close look at the figure of the Period Table (the first figure of this lesson). Can you spot the column labeled "3A"? Can you spot the column labeled "3B"? Notice that the smallest atom in the "3B" column has Z = 21 (Scandium, Sc), while the smallest atom in the "3A" column has Z = 5 (Boron, B). (You need to note that there is an alternate way to name 3A elements; they can also be referred to as group 13 since these elements are in the 13th column of the Periodic Table.) Therefore, it obviously makes sense to discuss the 3A column first. Let's figure out how many valence electrons atoms in the 3A column have.
|
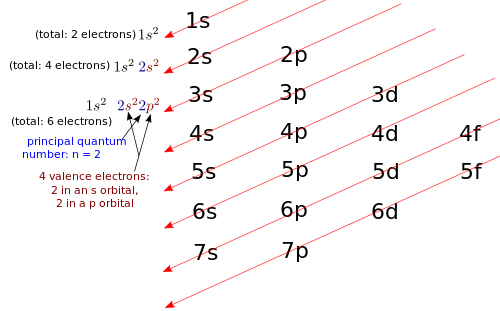
Example 6 – Boron Write the electron configuration for boron (B). Solution: The Periodic Table tells you that the atomic number for boron is Z = 5, thus boron has 5 protons. Neutral boron will also have 5 electrons. You need to write the electron configuration for an atom with 5 electrons. As pictured below, the diagonal rule applied to boron (B). Non-valence electrons: 1s2. Therefore, the electron configuration for B: 1s22s22p1. What is the highest principal quantum number that you see in boron's electron configuration? It's n = 2, so all electrons with n = 2 are valence electrons, and all electrons with n < 2 are non-valence electrons. Both the electron in the 2p orbital and the electrons in the 2s orbital are valence electrons. Boron has 2 valence electrons in an s orbital, and 1 valence electron in a p orbital, for a total of 3 valence electrons. |
|
Example 7 – Aluminum Write the electron configuration for aluminum (Al). Solution: The Periodic Table tells you that the atomic number for aluminum is Z = 13; thus neutral aluminum has 13 protons and 13 electrons. You need to write the electron configuration for an atom with 13 electrons. Therefore, the electron configuration for Al: 1s22s22p63s23p1. What is the highest principal quantum number that you see in aluminum's electron configuration? It's n = 3, so all electrons with n = 3 are valence electrons, and all electrons with n < 3 are non-valence electrons. Both the electron in the 3p orbital and the electrons in the 3s orbital are valence electrons. Aluminum has 2 valence electrons in an s orbital, and 1 valence electron in a p orbital, for a total of 3 valence electrons. |
From Example 6 and Example 7, we have:
- Boron has 2 valence electrons in an s orbital and 1 valence electron in a p orbital
- Aluminum has 2 valence electrons in an s orbital and 1 valence electron in a p orbital
In fact, all elements in the 3A column of the Periodic Table have 2 valence electrons in an s orbital and 1 valence electron in a p orbital. That's a total of 3 valence electrons for atoms in the 3A column. Again, the chemical properties of 3A elements are similar, because they have the same number and type of valence electrons.
Group 4A-8A Continue to Add p Electrons to the Outermost Energy Level
[edit | edit source]By now, you may have noticed a pattern relating the number of valence electrons to the column number. Group 1A elements have 1 valence electron. Group 2A elements have 2 valence electrons. Group 3A elements have 3 valence electrons. Group 4A elements have… well, we haven't looked at them yet, but what would you guess? It's pretty obvious. Group 4A elements have 4 valence electrons. Similarly, Group 5A elements have 5 valence electrons. In fact, the pattern continues all the way up to Group 8A elements, which have 8 valence electrons. Let's take a look at a few examples in order to figure out exactly what types of valence electrons are involved. First, we'll consider a Group 4A element.
|
Example 8 – Carbon Write the electron configuration for carbon (C). Solution: The Periodic Table tells you that the atomic number for carbon is Z = 6, thus neutral carbon has 6 protons and 6 electrons. You need to write the electron configuration for an atom with 6 electrons. Illustrated below, the diagonal rule applied to carbon (C). Non-valence electrons: 1s2. Therefore, the electron configuration for C: 1s22s22p2. What is the highest principal quantum number that you see in carbon's electron configuration? It's n = 2, so all electrons with n = 2 are valence electrons, and all electrons with n < 2 are non-valence electrons. Both the electrons in the 2p orbitals and the electrons in the 2s orbital are valence electrons. Carbon has 2 valence electrons in an s orbital, and 2 valences electron in p orbitals, for a total of 4 valence electrons. |
Now let's consider a Group 5A element.
|
Example 9 – Nitrogen Write the electron configuration for nitrogen (N). Solution: The Periodic Table tells you that the atomic number for nitrogen is Z = 7, neutral nitrogen has 7 protons and 7 electrons. You need to write the electron configuration for an atom with 7 electrons. Therefore, the electron configuration for N: 1s22s22p3. What is the highest principal quantum number that you see in nitrogen's electron configuration? It's n = 2, so all electrons with n = 2 are valence electrons, and all electrons with n < 2 are non-valence electrons. Both the electrons in the 2p orbitals and the electrons in the 2s orbital are valence electrons. Nitrogen has 2 valence electrons in an s orbital, and 3 valence electrons in p orbitals for a total of 5 valence electrons. |
As a final example, let's take a look at a Group 6A element (or Group 16).
|
Example 10 – Oxygen Write the electron configuration for oxygen (O). Solution: The Periodic Table tells you that the atomic number for oxygen is Z = 8; neutral oxygen has 8 protons and 8 electrons. You need to write the electron configuration for an atom with 8 electrons. Therefore, the electron configuration for O: 1s22s22p4. What is the highest principal quantum number that you see in oxygen's electron configuration? It's n = 2, so all electrons with n = 2 are valence electrons, and all electrons with n < 2 are non-valence electrons. Both the electrons in the 2p orbitals and the electrons in the 2s orbital are valence electrons. Oxygen has 2 valence electrons in an s orbital, and 4 valence electrons in p orbitals, for a total of 6 valence electrons. |
So let's summarize what we know so far:
- Group 1A elements have 1 valence electron in an s orbital
- Group 2A elements have 2 valence electrons in an s orbital
- Group 3A elements have 2 valence electrons in an s orbital and 1 valence electron in a p orbital
- Group 4A elements have 2 valence electrons in an s orbital and 2 valence electrons in p orbitals
- Group 5A elements have 2 valence electrons in an s orbital and 3 valence electrons in p orbitals
- Group 6A elements have 2 valence electrons in an s orbital and 4 valence electrons in p orbitals
- Group 7A elements have 2 valence electrons in an s orbital and 5 valence electrons in p orbitals
- Group 8A elements have 2 valence electrons in an s orbital and 6 valence electrons in p orbitals
Notice that, after column 3A, each column one step further to the right has one additional valence p electron. Group 4A elements have one more valence p electron than Group 3A elements. Similarly, Group 5A elements have one more valence p electron than Group 4A elements. But what happens when you reach Group 8A elements? Why does the Periodic Table end at column 8A? Let's think about that carefully. Group 8A elements have 6 valence electrons in p orbitals. In the last chapter, you learned that the maximum number of p electrons at any energy level is 6. Therefore, there couldn't be a "9A" column, because a "9A" column would have 7 p electrons in the valence energy level, which is impossible.
The fact that Group 8A elements have completely filled valence s sublevel and p sublevel is important in terms of their chemical properties. Group 8A elements are called noble gases. They are all gases, and they are not very reactive at all.
Lesson Summary
[edit | edit source]- All known elements are organized into the Periodic Table in such a way that elements in the same column have similar chemical properties.
- Only the highest energy electrons (valence electrons) are involved in chemical reactions. Therefore, it is only these high-energy electrons that are important in determining an elements chemical properties.
- Two different elements are likely to have similar chemical properties when they have the same number of valence electrons.
- Elements with the same number of valence electrons are found in the same column of the Periodic Table.
- Elements with the same valence shell are found in the same row on the Periodic Table.
- All elements in the first column of the Periodic Table have 1 valence electron in an s orbital. These elements are known as Group 1A metals or alkali metals.
- All elements in the second column of the Periodic Table have 2 valence electrons in an s orbital. These elements are known as Group 2A metals or alkaline earth metals.
- All elements in column 3A of the Periodic Table have 2 valence electrons in an s orbital and 1 valence electron in a p orbital.
- All elements in column 4A of the Periodic Table have 2 valence electrons in an s orbital and 2 valence electrons in p orbitals… etc.
- Column 8A has 2 valence electrons in an s orbital and 6 valence in p orbitals. Since any given energy level can have at most 6 p electrons, column 8A elements have a filled p sublevel. Therefore, they are inert (non-reactive), because they are unlikely to either gain or lose electrons. Group 8A elements are called noble gases.
Review Questions
[edit | edit source]- Take a look at the Periodic Table. How would you describe it? Why do you think it has such a funny shape?
- Can you suggest how elements in the same column of the Periodic Table might be similar?
- Choose the correct statement.
- (a) Mg has only 1 valence electron in an s orbital
- (b) F has only 1 valence electron in an s orbital
- (c) O has only 1 valence electron in an s orbital
- (d) Kr has only 1 valence electron in an s orbital
- Circle the appropriate element for each blank.
- (a) ____________ (Mg/N) has 2 valence electrons in an s orbital, and 3 valence electrons in p orbitals.
- (b) ____________ (As/B) has 2 valence electrons in an s orbital, and 3 valence electrons in p orbitals
- (c) ____________ (Cl/P/Li) has 2 valence electrons in an s orbital, and 5 valence electrons in p orbitals
- (d) ____________ (Al/Li/Na) has 1 valence electron in a p orbital
- Choose the correct statement.
- (a) Group 1A elements have a total of 3 valence electrons
- (b) Group 5A elements have a total of 2 valence electrons
- (c) Group 7A elements have a total of 4 valence electrons
- (d) Group 8A elements have a total of 8 valence electrons
- (e) Group 2A elements have a total of 5 valence electrons
- (f) Group 1A elements have a total of 3 valence electrons
- Fill in the blanks.
- (a) N has ___ valence electrons in an s orbital
- (b) N has ___ valence electrons in p orbitals
- (c) N has a total of ___ valence electrons
- (d) Ca has ___ valence electrons in s orbitals
- (e) Ca has ___ valence electrons in p orbitals
- (f) Ca has a total of ___ valence electrons
- Decide whether each of the following statements is true or false.
- (a) K has 1 valence electron in an s orbital
- (b) Ge has 2 valence electrons in an s orbital
- (c) Se has 4 valence electrons in p orbitals
- (d) B has 3 valence electrons in p orbitals
- (e) F has 2 valence electrons in an s orbital, and 7 valence electrons in p orbitals
- (f) Ca has a total of 4 valence electrons
- Match the element to its valence electrons.
(a) Sr i. a total of 8 valence electrons (b) I ii. a total of 2 valence electrons (c) Ne iii. a total of 5 valence electrons (d) N iv. a total of 7 valence electrons
- Fill in the blanks.
- (a) Ba has __ valence electron(s) in an s orbital, and __ valence electron(s) in p orbitals
- (b) Sn has __ valence electron(s) in an s orbital, and __ valence electron(s) in p orbitals
- (c) S has __ valence electron(s) in an s orbital, and __ valence electron(s) in p orbitals
- (d) Po has __ valence electron(s) in an s orbital, and __ valence electron(s) in p orbitals
- (e) Na has __ valence electron(s) in an s orbital, and __ valence electron(s) in p orbitals
- List all of the elements with exactly 2 valence electrons in p orbitals.
- An element has 2 valence electrons in an s orbital and 4 valence electrons in p orbitals. If the element is in the second row of the Periodic Table, which element is it?
- An element has 2 valence electrons in an s orbital and 6 valence electrons in p orbitals. If the element is in the same row as In, which element is it?
Vocabulary
[edit | edit source]- alkali metals
- Group 1A metals. These are elements found in the first column of the Periodic Table, excluding hydrogen.
- alkaline earth metals
- Group 2A metals. These are elements found in the second column of the Periodic Table.
- chemical properties
- The ways in which an element reacts with another element or compound.
- noble gases
- Group 8A elements. These are elements found in the eight column of the Periodic Table. They are inert, which means that they are very non-reactive.
- non-valence electrons
- All electrons in atom which are not valence electrons. Non-valence electrons are not important in determining an element's chemical properties because they rarely get involved in chemical reactions.
- valence electrons
- The electrons in an atom with the highest value of n (the electrons in the highest energy level).
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License