High School Chemistry/Orbital Configurations
In the Electron Configurations of Main Group Elements lesson, you learned a little bit about valence electrons. You saw how the number and type of valence electrons are important in determining the chemical properties of a particular element. Group 1A metals were highly reactive, because they have a strong tendency to lose their single valence s electrons. Group 2A metals are reactive as well, but less so, because they had 2 valence s electrons. Finally, Group 8A elements were inert (not reactive at all), because they had completely filled valences and p sublevels, meaning they could neither lose nor gain electrons very easily. Now you might be wondering why we didn't talk much about the chemical properties of the elements in columns 4A-7A. It turns out that understanding the behavior of these elements requires a bit more information. Specifically, we need to know how the electrons fill up the p orbitals. Carbon, for instance, is a Group 4A element, so it has 2 valence s electrons, and 2 valence p electrons. Obviously, the 2 valence s electrons are paired together in the s orbital, but what about the 2 valence p electrons? Are the valence p electrons paired in a single p orbital, or are they each in their own p orbital (remember, there are a total of three p orbitals that the valence p electrons could be found in). What about nitrogen? Nitrogen is a Group 5A element, so it has 2 valence s electrons, and 3 valence p electrons. Again, the 2 valence s electrons must be paired in the s orbital, but what about the 3 valence p electrons? Are two of them paired in a single p orbital, or do all three have their own p orbitals?
Lesson Objectives
[edit | edit source]- Draw orbital diagrams.
- Define Hund's Rule.
- Use Hund's Rule to decide how electrons fill sublevels with more than one orbital.
Orbital Representation
[edit | edit source]Before we discuss the order and manner in which the orbitals in a p sublevel are filled, we have to introduce a symbolic notation that scientists use to show orbital filling. Frequently, scientists will use boxes to represent orbitals.
The figure below shows a set of boxes for orbitals up to the n = 2 energy level. The boxes are depicting electron orbitals for the n = 1 and n = 2 energy levels. Notice that for n = 1, there is a single s orbital, while for n = 2 there is one s orbital and three p orbitals.
When a "spin-up" electron is present in an orbital, scientists draw an upward pointing arrow in that orbital's box. This leads to what is known as an orbital diagram. For example, hydrogen has a single electron in the 1s orbital. If that single electron were a spin-up (ms = +1/2), the orbital diagram for hydrogen would be as illustrated below:
When a "spin-down" electron is present in an orbital, scientists draw a downward pointing arrow in that orbital's box. Helium, for instance, has two electrons in the 1s orbital. We know that one of these electrons must be "spin-up" and the other must be "spin-down", so the orbital diagram for helium would be as shown below:
By comparing the first two orbital representation figures, it should be fairly obvious to you which electrons are paired and which are unpaired. That is one of the advantages of an orbital diagram. An orbital diagram is a clear way of showing exactly how many paired and unpaired electrons there are in a particular atom's electronic configuration.
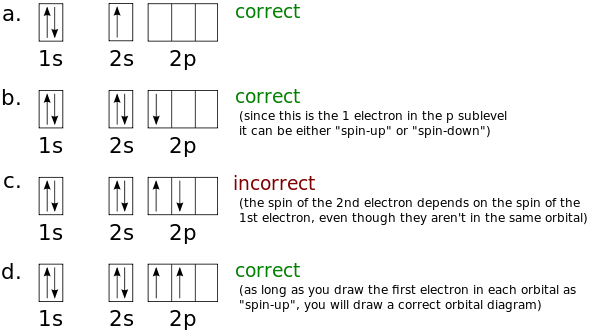
Now you might be wondering about the unpaired electrons – how do you know whether to draw them as "spin-up", or "spin-down". Technically speaking, the first electron in a sublevel (1s, 2s, 2p, etc) could be either "spin-up" or "spin-down". In other words, for hydrogen (1s1), you could draw the arrow in the 1s orbital box pointing either up or down. Similarly, for boron (1s22s22p1), you could draw the arrow in the 2p orbital box pointing either up or down. Once you've chosen the spin of the first electron in a sublevel, though, the spins of all of the other electrons in that sublevel depend on the spin you chose for the first. To avoid confusion, scientists "always draw the first electron in an orbital as 'spin-up'". If you stick with this rule, you'll never get into trouble.
If you insist on breaking this rule, you might draw an orbital diagram that is incorrect, as shown in the figure below. By convention, scientists usually draw all unpaired electrons as "spin-up". This prevents them from drawing incorrect orbital diagrams like the one shown in (c). In the next section, you'll learn that the orbital diagram in (c) is incorrect because it does not obey Hund's Rule.
Notice that by drawing the first electron in each orbital as "spin-up", all of the unpaired electrons in the sublevel have the same spin, even if they are in different orbitals! This is due to a principle known as Hund's Rule. We'll discuss Hund's Rule in more detail in the next section.
Orbitals in p Sublevel Fill Individually Before Pairing
[edit | edit source]Previously we learned that the energy of an electron in any given orbital depends on the energy level of the orbital (which is determined by the principal quantum number, n) and the sublevel of the orbital (s, p, d, etc. which is determined by the azimuthal quantum number: ℓ). We also learned that according to the Aufbau principle, electrons will fill the lowest energy orbitals first, and then move up to higher energy orbitals only after the lower energy orbitals are full. If you think carefully, though, you'll realize that there's still a problem. Certainly, 1s orbitals should be filled before 2s orbitals, because the 1s orbitals have a lower value of n, and thus a lower energy. Similarly, 2s orbitals should be filled before 2p orbitals, because 2s orbitals have a lower value of ℓ (ℓ = 0), and thus a lower energy. What about the three different 2p orbitals? In what order do electrons fill the 2p orbitals? To answer this question, we need to turn to a principle known as Hund's Rule. Hund's Rule states that: (1) Every orbital in a sublevel is singly occupied before any orbital is doubly occupied. (2) All of the electrons in singly occupied orbitals have the same spin.
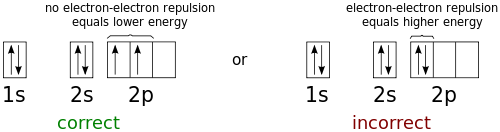
According to the first rule, electrons will always occupy an empty orbital before they pair up. This should make sense given what you know about electrons. Electrons are negatively charged and, as a result, they repel each other. Since electron-electron repulsion raises the energy of the electrons involved, electrons tend to minimize repulsion (and thus minimize their energies) by occupying their own orbital, rather than sharing an orbital with another electron. Take a look at the figure below.
Notice how the two 2p electrons in the orbital diagram on the left are in separate orbitals, while the two 2p electrons in the orbital diagram on the right are sharing a single orbital. The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule, meaning that there is less electron-electron repulsion and, as a result, the electrons have lower energies (remember, electrons always minimize their energies).
The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Notice how all three 2p electrons in the orbital diagram on the left are in separate orbitals, while two of the three 2p electrons in the diagram on the right are sharing a single orbital. The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule. Again, this means that there is less electron-electron repulsion and, as a result, the electrons have lower energies.
The figure below shows possible electron configurations for an atom with four 2p electrons. This time, two of the electrons have no choice – they must pair up. The other two electrons, however, could either pair up, as shown in the orbital diagram on the right, or occupy their own orbitals, as shown in the orbital diagram on the left. Which do you think is correct? Obviously, the orbital diagram on the left, because it minimizes electron-electron repulsion. The orbital diagram on the left is also the orbital diagram, which follows Hund's Rule, since all orbitals are singly occupied before any are doubly occupied. The orbital diagram on the right does not follow Hund’s Rule, since the first two orbitals are doubly occupied before the third is singly occupied.
While it's easy to understand why electrons would occupy empty orbitals before pairing up, it’s a lot harder to understand why unpaired electrons in different orbitals must all have the same spin. Electron spins in different orbitals align (all point in the same direction), because spins, which are aligned have lower energy than spins which are not aligned. Notice that as long as you always draw the first electron in an orbital as "spin-up" you will always draw spins which are aligned. Refer back to the figure illustrating unpaired electrons as "spin-up". Now that you know Hund's Rule, it should be obvious why the orbital diagram (c) is incorrect – the two electrons in the singly occupied 2p orbitals have different spins, and thus this orbital diagram does not obey Hund's Rule. Compare the orbital diagram (c) to the orbital diagram (d). The orbital diagram in 4.d does obey Hund's Rule, because the two electrons in the singly occupied 2p orbitals have the same spin.
Nitrogen Has Three Unpaired Electrons
[edit | edit source]Now that we know about orbital diagrams and Hund's rule, we can begin to explain the chemistry of the elements in Groups 4A through 7A. Let's take a look at nitrogen as an example.
|
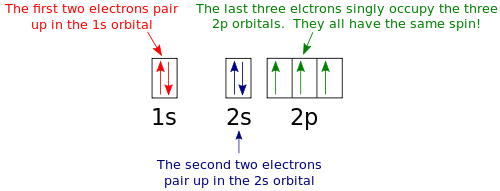
Example 1 Draw the orbital diagram for nitrogen. Solution: First, we need to write the electron configuration for nitrogen just as we did previously, which gives 1s22s22p3. To draw the orbital diagram we will write the following: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in the 2s orbital. That leaves 3 electrons, which must be placed in the 2p orbitals. According to Hund’s Rule, all orbitals will be singly occupied before any is doubly occupied. Therefore, we know that each p orbital gets one electron. Hund's Rule also tells us that all of the unpaired electrons must have the same spin. Keeping with convention, we draw all of these electrons as "spin-up", which gives: |
Orbital diagrams can help you to make predictions about the ways in which certain elements will react, and the chemical "compounds" or "molecules" that different elements will form. We aren't ready to discuss compounds or molecules yet. The principles that you're learning now will help you to understand the behavior of all chemicals, from the most basic elements like hydrogen and helium, to the most complex proteins (huge biological chemicals made of thousands of different atoms bound together) found in your body.
Lesson Summary
[edit | edit source]- Orbital diagrams are drawn by representing each orbital as a box, each "spin-up" electron in an orbital as an upward pointing arrow in the box, and each "spin-down" electron in an orbital as a downward pointing arrow in the box. You can only have two arrows in each box, and they must be pointing in opposite directions.
- Scientists use the convention that the first electron in any orbital is "spin-up", therefore, the first arrow in an orbital "box" should point up. Hund's Rule states:
- Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
- All of the electrons in singly occupied orbitals have the same spin.
- Electrons will occupy separate orbitals rather than pairing up, since this minimizes electron-electron repulsions, thereby minimizing energy. Electron spins in different orbitals within the same sublevel align because aligned spins have lower energy.
Review Questions
[edit | edit source]- Which of the following is a valid orbital diagram?
- Draw the orbital diagram for lithium (Li).
- Draw the orbital diagram for carbon (C).
- Draw the orbital diagram for fluorine (F).
- Draw the orbital diagram for oxygen (O). Use it to answer the following questions:
- (a) an oxygen atom has ___ unpaired valence electrons
- (b) an oxygen atom has ___ paired valence electrons
- (c) an oxygen atom has ___ paired non-valence electrons
- (d) an oxygen atom has ___ unpaired non-valence electrons
- Draw the orbital diagram for neon, Ne. Use it to answer the following questions:
- (a) a neon atom has ___ unpaired valence electrons
- (b) a neon atom has ___ paired valence electrons
- (c) a neon atom has ___ paired non-valence electrons
- (d) a neon atom has ___ unpaired non-valence electrons
- Decide whether each of the following statements is true or false.
- (a) Every orbital in a sublevel is doubly occupied before any orbital is singly occupied.
- (b) Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
- (c) All electrons in singly occupied orbitals have the same spin.
- (d) The two electrons in a single orbital have the same spin.
- (e) All electrons in singly occupied orbitals have different spins.
- (f) The two electrons in a single orbital have different spins.
- Draw the orbital diagram for phosphorus P.
- Draw an orbital diagram for silicon, Si. Use it to answer the following questions:
- (a) a silicon atom has ___ unpaired valence electrons
- (b) a silicon atom has ___ paired valence electrons
- (c) a silicon atom has ___ paired non-valence electrons
- (d) a silicon atom has ___ unpaired non-valence electrons
- Draw an orbital diagram for Mn. Use it to determine the total number of unpaired electrons in an Mn atom.
Vocabulary
[edit | edit source]- Hund's Rule
- Every orbital in a sublevel is singly occupied before any orbital is doubly occupied. All of the electrons in singly occupied orbitals have the same spin.
- orbital diagram
- Orbital diagrams are drawn by representing each orbital as a box, each "spin-up" electron in an orbital as an upward pointing arrow in the box, and each "spin-down" electron in an orbital as a downward pointing arrow in the box.
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License