Planet Earth/3e. The Periodic Table and Electron Orbitals
Electrons: how atoms interact with each other
[edit | edit source]If it was not for electrons inside atoms, atoms would never bond or interact with each other to form molecules, crystals and other complex materials. Electrons are extremely important in chemistry because they determine how atoms interact with each other. It is no wonder that the Periodic Table of Elements, found in most science classrooms is displayed rather than the more cumbersome Chart of the Nuclides, since the Periodic Table of Elements organizes elements by the number of protons and electrons, rather than the number of protons and neutrons.


As discussed previously, electrons are wayward subatomic particles that can increase their energy states and even leave atoms altogether to form plasma, which is also called electricity. Electricity is the flow of free electrons which can move near the speed of light across conducting material, like metal wires. In this next section we will look in detail at how electrons are arranged within atoms in orbitals. However, remember that highly excited atoms bombarded with high levels of electromagnetic radiation, such as increasing temperatures and high pressures, electrons can leave atoms, while at very cold temperatures, near absolute zero Kelvin electrons will be very close to the nucleus of atoms forming Bose–Einstein condensate. When we think of temperature (heat), what is really indicated is the energy states of electrons within the atoms of a substance, whether a gas, liquid or solid. The hotter a substance becomes the more vibrational energy electrons will have.
Electrons orbit around the nucleus at very fast speeds and under no discrete orbital path, but as an electromagnetic field called an orbital shell. The Heisenberg principle describes the impossible nature of measuring these electron orbital shells, because anytime a photon is used by a scientist to measure the position of an electron, it will move and change its energy level. There is always an uncertainty as to the exact location of an electron within the orbit around the atom’s nucleus. As such electron orbital shells are probability fields where an electron is likely to exist at any moment in time.
Negatively charged electrons are attracted to positively charged protons, such that equal numbers of electrons and protons are observed in most atoms.

Early chemists working in the middle 1800s knew of only a handful of elements, which were placed into three major groups based on how reactive they were with each other, the Halogens, Alkali Metals and Alkali Earths. By 1860, the atomic mass of many of these elements were reported, allowing the Russian scientist Dmitri Mendeleev to arrange elements based on their reactive properties and atomic mass.

While working on a chemistry textbook, Mendeleev stumbled upon the idea of each set of elements having increasing atomic mass, such that a set of Halogens would have elements of differing mass. Without knowing the underlying reason, Mendeleev organized the elements by their atomic number (number of protons), which is related to atomic mass and the number of electron orbitals, which is related to how reactive an element is to bonding with other elements. While these early Periodic Tables of Elements look nothing like our modern Periodic Table of Elements, they excited chemists to discover more elements. The next major breakthrough came with the discovery and wide acceptance of Noble gasses, which include Helium and Argon, which are the least reactive elements known.
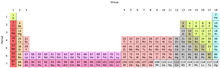
The Periodic Table of Elements
[edit | edit source]So how does an atom’s reactivity relate to its atomic mass? Electrons are attracted to the atomic nucleus in equal number to the number of protons, which is half the atomic mass. The more atomic mass, the more protons, and the more electrons will be attracted. However, electrons prefer to fill electron orbital shells in sets, such that an incomplete electron orbital shell will attract other electrons, despite there being an equal number of electrons to protons. If an atom has a complete set of electrons which matches the number of protons, it will be non-reactive, while elements that need 1 less or gain 1 more electron to fill an orbital set are the most reactive types of elements.
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| ↓ Period | ||||||||||||||||||||
| 1 | 1 H |
2 He | ||||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr | ||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe | ||
| 6 | 55 Cs |
56 Ba |
57 La |
58 Ce |
* |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
89 Ac |
90 Th |
** |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Uut |
114 Uuq |
115 Uup |
116 Uuh |
117 Uus |
118 Uuo |
| * Lanthanides | 59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu | |||||||
| ** Actinides | 91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr | |||||||
| Alkali metals2 | Alkaline earth metals2 | Lanthanides12 | Actinides12 | Transition metals2 |
| Poor metals | Metalloids | Nonmetals | Halogens3 | Noble gases3 |
1Actinides and lanthanides are collectively known as "Rare Earth Metals." 2Alkali metals, alkaline Earth metals, transition metals, actinides, and lanthanides are all collectively known as "Metals." 3Halogens and noble gases are also non-metals.
State at standard temperature and pressure
- those with atomic number in blue are not known at STP
- those with atomic number in red are gases at standard temperature and pressure (STP)
- those with atomic number in green are liquids at STP
- those with atomic number in black are solid at STP
Natural occurrence
- those with solid borders have isotopes that are older than the Earth (Primordial elements)
- those with dashed borders naturally arise from decay of other chemical elements and have no isotopes older than the earth
- those with dotted borders are made artificially (Synthetic elements)
Unknown properties
- those with a cyan background have unknown chemical properties.
The First Row of the Periodic Table of Elements (Hydrogen & Helium)
[edit | edit source]
The first row of the Periodic Table of Elements contains two elements, Hydrogen and Helium.
Hydrogen has 1 proton, and hence it attracts 1 electron. However, the orbital shell would prefer to contain 2 electrons, so hydrogen is very reactive with other elements, for example in the presence of oxygen, it will explode! Hydrogen would prefer to have 2 electrons within its electron orbital shell, but can’t because it has only 1 proton, so it will “steal” or “borrow” other electrons from nearby atoms if possible.
Helium has 2 protons, and hence attracts 2 electrons. Since 2 electrons are the preferred number for the first orbital shell, helium will not react with other elements, in fact it is very difficult (nearly impossible) to bond helium to other elements. Helium is a Noble Gas, which means that it contains the full set of electrons in its orbital shell.
The columns of the Period Table of Elements are arranged by the number of electrons within each orbital shell, and the atomic number (number of protons), is represented by rows.
The Other Rows of the Periodic Table of Elements
[edit | edit source]

The first row of the Periodic Table of Elements is the when the first 2 electrons fill the first orbital shell, called the 1s orbital shell. The second row is when the next 2 electrons fill in the 2s orbital shell, and 6 fill the 2p orbital shell. The third row is when the next 6 orbitals fill in the 3p orbital shell, and 2 fill the 4s orbital shell. The fourth row is when the next 10 orbitals fill in the 3d orbital shell, and 6 fill in the 4p orbital, and 2 fill in the 5s orbital.
Valence Electrons
[edit | edit source]A valence electron is an outer shell electron that is associated with an atom, but not completely filling the outer orbital shell, and as such is involved in bonding between atoms. The valence shell is the outermost shell of an atom. Elements with complete valence shells (noble gases) are the least chemically reactive, while those with only one electron in their valence shells (alkali metals) or just missing one electron from having a complete shell (halogens) are the most reactive. Hydrogen, has one electron in its valence shell but also is just missing one electron from having a complete shell has unique and very reactive properties.
The number of valence electrons of an element can be determined by the periodic table group, the vertical column in the Periodic Table of Elements. With the exception of groups 3–12 (the transition metals and rare earths), the columns identify by how many valence electrons are associated with a neutral atom of the element. Each s sub-shell holds at most 2 electrons, while p sub-shell holds 6, d sub-shells hold 10 electrons, followed by f which holds 14 and finally g which holds 18 electrons. Observe the first few rows of the Periodic Table of Elements to see how this works to determine how many valance electrons ( < ) are in each atom of a specific element.
| Element | # Electrons | 1s | 2s | 2p | 2p | 2p | # Valance
Electrons |
|---|---|---|---|---|---|---|---|
| Hydrogen | 1 | < | 1 | ||||
| Helium | 2 | <> | 0 | ||||
| Lithium | 3 | <> | < | 1 | |||
| Beryllium | 4 | <> | < | < | 2 | ||
| Boron | 5 | <> | < | < | < | 3 | |
| Carbon | 6 | <> | < | < | < | < | 4 |
| Nitrogen | 7 | <> | <> | < | < | < | 3 |
| Oxygen | 8 | <> | <> | <> | < | < | 2 |
| Fluorine | 9 | <> | <> | <> | <> | < | 1 |
| Neon | 10 | <> | <> | <> | <> | <> | 0 |
Notice that Helium and Neon have 0 valance electrons, which means that they are not reactive, and will not bond to other atoms. However, Lithium has 1 valance electron, if this 1 electron was removed, it would have 0 valence electrons, this makes Lithium highly reactive. Also notice that Fluorine just needs 1 more valence electron to complete its set of 2s and 2p orbitals, making Fluorine highly reactive as well. Carbon has the highest number of valence electrons in this set of elements, which will attract or give up 4 electrons to complete the set of 2s and 2p orbitals.
Understanding the number of valence electrons is extremely important in understanding how atoms form bonds with each other to form molecules. For example, the first column of elements containing Lithium on the periodic table all have 1 valence electron, and likely will bond to elements that need 1 valence electron to fill the orbital shell, such as elements in the fluorine column on the Periodic Table of Elements.
Some columns of the periodic table are given specific historical names. The first column of elements containing Lithium collectively are called the Alkali Metals (hydrogen a gas is unique and often not considered within Alkali Metals), the last column of the elements containing Helium all have 0 valence electrons, and are collectively called the Noble Gases. Elements under the Fluorine column require 1 valence electron to fill the orbital shell and are called the Halogens, while elements under Beryllium are called the Alkaline Earth Metals, and have 2 valence electrons. Most other columns are not given specific names (sometimes collectively called Transitional Metals), but can be used to determine the number of valence electrons, for example Carbon and elements listed below will have 4 valence electrons, while all elements listed under Oxygen will have 2 valence electrons. Notice that after the element Barium, there is an insert of two rows of elements, these are the Lanthanoids and Actinoids, which contain electrons in the 4s, 4p, 4d, 4f orbitals, for a possible total of 32 electrons, a little too long to include in a nice table, and hence often these elements are shown at the bottom of the Periodic Table of Elements.
A typical college class in chemistry will go into more detail on electron orbital shells, but it is important to understand how electron orbitals work, because the configuration of electrons determines how atoms of each element form bonds in molecules. In the next section, we will examine how atoms come together for form bonds, and group together in different ways to form the matter that you observe on Earth.
| Previous | Current | Next |
|---|---|---|
|
f. Chemical Bonds (Ionic, Covalent, and others means to bring atoms together). |
