Planet Earth/3f. Chemical Bonds (Ionic, Covalent, and Others)
There are three major types of bonds that form between atoms, linking them together into a molecule, Covalent, Ionic, and Metallic. There are also other ways to weakly link atoms together, because of the attractive properties related to the configuration of the molecules themselves, which includes Hydrogen bonding.
Covalent Bonds
[edit | edit source]
Covalent bonds are the strongest bonds between atoms found in chemistry. Covalent bonding is where two or more atoms share valence electrons to complete their orbital shells. The most-simple example of a covalent bond is found when two hydrogen atoms bond. Remember that each hydrogen atom has 1 proton, and 1 electron, however to fill the s1 orbital requires 2 electrons. Hydrogen atoms will group into pairs, each contributing an electron to the s1 orbital shell. Chemical hydrogen will be paired, which is depicted by the chemical formula H2. Another common covalent bond can be illustrated, by introducing oxygen with hydrogen. Remember that oxygen needs two valence electrons to fill its set of electron orbitals, hence it bonds to 2 hydrogen atoms, each having 1 valence electron to share between the atoms. H2O the chemical formula for ice or water is where 2 hydrogen atoms, each with an electron, bond with an oxygen atom that needs 2 electrons to fill its s2 p2 orbitals. In covalent bonds, atoms share the electron to complete the orbital shells, and because the electrons are shared between atoms covalent bonds are the strongest bonds in chemistry.

Oxygen for example, will pair up to share the 2 electrons (called a double bond), forming O2. Nitrogen does the same, pairing up to form N2, by sharing 3 electrons (called a triple bond). However, in the presence of nitrogen and hydrogen, the hydrogen will bond with nitrogen forming NH3 (ammonia) because it would require 3 electrons each from a hydrogen atom to fill all the orbitals. Carbon which has 4 valence electrons most often bonds with hydrogen to form CH4 (methane or natural gas), because it requires 4 electrons each from a hydrogen atom. Bonds that form by two atoms sharing 4 or more electrons are very rare.

The electrons shared equally between the atoms makes these bonds very strong. Covalent bonds can form crystal lattice structures, when valence electrons are used to link atoms together. For example, diamonds are composed of linked carbon atoms. Each carbon atom is linked to 4 other carbon atoms, which each share an electron between them. If the linked carbon forms a ring, rather than a lattice structure, the carbon is in the form of graphite (used at the end of pencils). If the linked carbon forms a lattice structure the crystal form is much harder, a diamond. Hence the only difference between graphite pencil lead and a valuable diamond is how the bonds between the carbon atoms are linked together in covalent bonds.
Ionic Bonds
[edit | edit source]
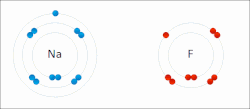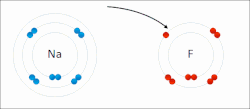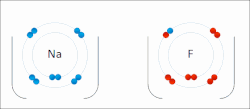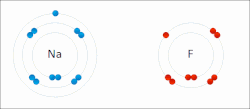
Ionic bonds are a weaker type of bond between atoms found in chemistry. Ionic bonding is where one atom gives a valence electron to complete another atom’s orbital shell. For example, lithium has a single valence electron, and would like to get rid of it, so it will give or contribute the electron to an atom of fluorine, which needs an extra valence electron. In this case the electron is not shared by the two atoms, however, when lithium gives away its valence electron, it becomes positively charged because it has fewer electrons than it has of protons. While fluorine, will have more electrons than protons, and will be negatively charged. Because of this charge, the atoms will be attracted together. Atoms that have different numbers of protons and electrons are called ions. Ions can be positively charged like Lithium, which are called cations or negatively charged like Fluorine which are called anions.
An excellent example of ionic bonding you have encountered is table salt, which is composed of Sodium (Na) and Chloride (Cl). Sodium has one extra valence electron that it would like to give away, and Chloride is looking to pick up an extra electron to fill its orbital, this results in Sodium (Na) and Chloride (Cl) ionically bonding to form table salt. However, the bonds in salt are easy to break, since they are held not by sharing electrons, but by their different charges. When salt is dropped in water, the pull of the water molecules can break apart the sodium and chloride, resulting in Sodium and Chloride ions (the salt is dissolved within the water). Often chemical formulas of ions are expressed as Na+ and Cl− to depict the charge, where + sign indicates a cation and − sign indicates an anion. Sometimes an atom will give away or receive two or more electrons, for example Calcium will often give up two electrons, resulting in the cation Ca2+.
The difference between covalent bonds and ionic bonds is that in covalent bonds the electrons are shared between atoms, while in ionic bonds the electrons are given or received between atoms. A good analogy to think of is friendship between two kids. If the friends are sharing a ball, by passing it between each other, they are covalently bonded to each other since the ball is shared equally between them. However, if one of the friends has an extra ice cream cone, and gives it to their friend, they are ionically bonded to each other.
Some molecules can have both ionic and covalent bonds. A good example of this is with a common molecule Calcium Carbonate CaCO3. The carbon atom is covalently bonded to three oxygen atoms, which means that it shares electrons between the carbon and oxygen atoms. Typically carbon only covalently bonds to two oxygen atoms (forming carbon dioxide CO2), each sharing two electrons, for a total of 4. However, in the case of carbonate, three oxygen atoms are bonded to the carbon, with 2 sharing 1 electron, and one sharing 2 electrons, this results in 2 extra electrons. Hence CO3-2 has two extra electrons that it would like to give away, and is negatively charged. Calcium atoms have 2 electrons more than a complete shell, and will lose these electrons resulting in a cation with a positive charge of +2, Ca+2. Hence the ions CO3-2 and Ca+2 have opposite charges that will bond together and form CaCO3, calcium carbonate, a common molecule found in limestones and shelled organisms that live in the ocean. Unlike salt, CaCO3 does not readily dissolve in pure water, as the ionic bonds are fairly strong, however if the water is slightly acidic, CaCO3, calcium carbonate will dissolve.
A solution is called an acid when it has an abundance of ions of hydrogen within the solution. Hydrogen ions, lose 1 electron, forming a cation H+. When there is an excess of hydrogen ions in a solution, these will break ionic bonds by bonding to anions. For example, in CaCO3, the hydrogen ions can form bonds with the CO3-2, forming HCO3- ions, called bicarbonate, dissolving the CaCO3 molecule. Acids break ionic bonds, by introducing ions of hydrogen, which can dissolve molecules that form these ionic bonds. Note that a solution with an abundance of anions, such as OH-, can also break ionic bonds, and these are called bases. So a basic solution, is one with an excess of anions. In this case the calcium will form a bond with the OH- anion, forming Ca(OH)2, calcium hydroxide, which in a solution of water is known as limewater.
The ratio of ions of H+ and OH- is measured in pH, such that a solution with a pH of 7 has equal numbers of H+ and OH- ions, while acidic solutions have pH less than 7, with more H+ cations, while basic solutions have pH more than 7, with more OH- anions.
Metallic bonding
[edit | edit source]Metallic bonding is a unique feature of metals, and can be described as a special case of ionic bonding involving the sharing of free electrons among a structure of positively charged ions (cations). Materials composed of metallic bonded atoms exhibit high conductivity of electricity, as electrons are free to pass between atoms across its surface. This is why electrical wires are composed of metals like copper, gold, and iron, since they can conduct electricity across their surface, since electrons are shared evenly between many atomic bonds. Material composed of metallic bonding also have a metallic luster or shine, and can be more ductile (bend) easily because of the flexibility of these bonds.

Metallic bonds are susceptible to oxidation. Oxidation is a type of chemical reaction in which metallic bonded atoms lose electrons to an oxidizing agent (most often atoms of oxygen), resulting in metallic atoms becoming bonded covalently with oxygen. For example, iron (Fe) which can be either cations of Fe2+ (Iron-II) or Fe3+ (Iron-III) lose electrons and can receive these missing electrons with oxygen (O-2), which has two extra electrons in its orbitals, resulting in a series of molecules called iron oxides such as Fe2O3. This is why metals, such as iron, rust or corrode and silver tarnishes over time. These metallic bonds react with the surrounding oxygen by gaining extra electrons from them. Oxygen is common in the air, within water and within acidic solutions (corrosive solutions), and the only way to prevent oxidation in metals is to limit the exposure to oxygen (and other atoms with an excess of electrons like fluorine).
When electrons are gained the reactive is called a reducing reaction, and is the opposite of oxidation. Collectively these types of chemical reactions are called "Redox" reactions and they form an important aspect in chemistry. Furthermore, the transfer of elections in oxidation-reduction reactions are a useful way to store excessive electrons (electricity) in batteries.
Hydrogen bonding
[edit | edit source]
Covalent, Ionic and Metallic bonding all require the exchange of electrons between atoms, and hence are fairly strong bonds, with covalent bonds being the strongest type of bond. However, molecules themselves can become polarized because of the arrangement of the atoms, such that a molecule can have a more positive and more negative side. This frequently happens with molecules containing hydrogen atoms bonded to larger atoms. These types of bonds are very weak and easily broken, but produce very important aspects in the chemistry of water and organic molecules essential for life. Hydrogen bonds form within water and is the reason for the expansion in volume between liquid water and solid ice. Water is composed of oxygen bonded to two hydrogen atoms covalently (H2O). The distribution of these two hydrogen atoms contribute an electron to the p2 orbitals, which require 6 electrons. Hence the two hydrogen atoms are pushed toward each other slightly because of the pair of electrons in the first p2 orbital, forming a “mouse-ear” like molecule. These two hydrogen atoms are more positively charged and give the molecule a slight positive charge at the hydrogen atom side compared to the other side of the oxygen atom which lacks a hydrogen atom. Hence water molecules oriented themselves with weak bonds between the positively charged hydrogen atoms and the open space negative charge side of the atoms. Hydrogen bonds are best considered an electrostatic force of attraction between hydrogen (H) atoms which are covalently bound to more electronegative atoms such as oxygen (O) and nitrogen (N). Hydrogen bonds are very weak, but provide important bonds in living organism, such as the bonds within the helix in the double helix structure of DNA (Deoxyribonucleic acid), and hydrogen bonds are important in capillary forces with water transport in plant tissue and blood vessels, as well as hydrophobic (water repelling) and hydrophilic (water attracting) organic molecules in cellular membranes.

Hydrogen bonding is often considered as a special type of the weak Van der Waals molecular forces which cause the attraction or repulsion of electrostatic interacts between electronically charged or polarized molecules. These forces are weak, but play a role in making some molecules more “sticky” than other molecules. As you will learn later on, water is a particularly “sticky” molecule because of these hydrogen bonds.
| Previous | Current | Next |
|---|---|---|
|
f. Chemical Bonds (Ionic, Covalent, and others means to bring atoms together). |
