Structural Biochemistry/Aging and Metabolic Control Analysis
Introduction
[edit | edit source]Aging is when an organism, such as a cell, has accumulated an excess amount of damage over its lifetime. The resulting damage ends up having an effect on the overall survival and status of the organism. As an organism ages, “degradation of their outputs leads to functional decline and death as a result of aging” (1). This degradation of outputs is related to the metabolic history of the cell which affects the cell’s function.
The concept of aging has led to the accepted idea that aging is due to the accumulation of damage an organism as acquired over its lifetime resulting in the inability to protect, maintain, and repair itself. Experiments are being conducted that are trying to determine what kind of damage, related to aging, contributes to the loss of function for an organism. This is difficult as there are many factors dealing with damage that can vary across models and individuals such as amount of damage, type of tissue, age, and simply the kind of organism in question. To begin, exactly what does an organism have to do in order to survive? First, the organism must be able to find food, shelter, and to fight off infections or predators. The organism must be able to avoid death. Even if these factors are eliminated, death and loss of function still occur with age. Nonetheless, no genes have evolved to cause death. An aging organism is said to reduce the genetic contribution of an individual for the next generation. In other words, it is disadvantageous.
In Murphy’s paper, “Control Theory of Aging,” he states that “the genome and how it is expressed constrain mortality and life span.” However, there is still a problem with this idea for life span varies across all organisms, even if they are genetically similar. It is concluded that it is indeed a combination of genetic determination, variation in the environment, and other events that occur in the organism’s life that contribute to death at the age to which the aforementioned factors can affect. As a result of a lifetime of low-dose exposure to external factors such as ultraviolet radiation and gamma-ray irradiation, the body's ability to carry out homeostatic mechanisms begins to fail and aging becomes apparent. It also has been proposed that aging is a reflection of cellular senescence, an irreversible halt in the cell's ability to self-replicate and grow.
An obstacle involves mutations and environmental interventions that hinder a number of functions which proves difficult to see what the cause for aging is. As mentioned before, there can be a variety of reasons why an organism may have died. It is important to see that one must look across all the possible biochemical and physiological entities and to observe each one independently. This will help narrow down the cause responsible.
Hierarchical Framework for Considering Organismal Aging
[edit | edit source]The top level of hierarchy is the organism’s functions and a set of physiological systems that are involved in the interaction with one another and the surrounding environment. Each system interacts with each other and also the environment in some manner by inputs and outputs.

In the figure above, for simplicity, interacting physiological systems are shown as two separate systems. They are affected by inputs from the environment and from other systems. For example, the mortality of an organism increases as the various outputs of its systems diminish over time due to the aging of the organism. Damage to the organism can also cause dysfunction which can result in inappropriate system outputs.
1. Dysfunction of Physiological Systems
[edit | edit source]In general, systems within an organism usually decline with aging. However, it is clear that mortality in different species is not the same. One theory involves natural selection that may influence an organism’s process of aging. The surviving generations of organisms can display characteristics that can be conserved by evolution. This hints at the possibility of a similar aging process in different organisms from flies, mice, yeast, and worms. Nonetheless, the biological age is not reliable as this also varies across species. Other aspects of aging can be organ specific and manifest in the appearance of skin aging. For an independent system, it is difficult to know for certain whether it is the effect of defective inputs or intrinsic damage to that system that result in the cell’s dysfunction. All the aforementioned provides reasons why it is difficult for scientists to pinpoint whether if it is the decline of all physiological systems or just one that results in aging and eventual death of the organism.
2. Changes in Cell Number, Function, And Phenotype
[edit | edit source]Cells can undergo many changes but the changes that affect the functional outputs of physiological systems will influence aging. Dysfunction of a system is due to the decrease in number of cells and their outputs. According to Murphy, “the changes to cells are caused by their metabolic history and are due to nonspecific damage and to changes in signaling pathways and gene expression. These in turn lead to effects on cell function and on cell number.” Tissues undergo many changes as the organism ages. The aging causes a change in the cell number. The decline in cell number results in a disruption of mechanisms that maintain the cell.
In some mitotic tissues can replenish lost cells from other differentiated cells. But as the organism ages, mammalian stem cells are less effective at replenishing lost cells. In order to determine whether a physiological system is impaired by the decline in cell number, it is crucial to record the cell loss or gain that occurs before and after the impairing is seen in the system. An example of a decline in cell function includes the synaptic transmission in neurons and contractions of musculoskeletal motor units. In mammals, studies have shown that the increase in age results in a decline in motor and neurological function due to decreased numbers of synaptic connections and conductions.
3. Nutrient Sensing
[edit | edit source]Longevity linked to animals under dietary restrictions becomes the organism's response to food availability and their reaction to nutritional changes in the environment. These changes are measured with its energy at its cellular level when it's activated. Changes in feeding patterns and foraging behavior are also indicators of its adaptive phases. These nutrient-sensing pathways also gives the organism time to detect and respond to those changes in its resource availability. However, because this kind of longevity depends on daf-16, which means the nutrient sensing of these neurons aren't necessary.
Cell Metabolic History
[edit | edit source]We now look at the individual cell where cell dysfunction and death are attributes to cell’s metabolic history. These attributes will affect system function and thus morality of the organism. The initial state of a cell is due to its genome which results in developmental history, physical niche occupied within the organism, and epigenetic factors affecting genomic expression.
A cell’s metabolic history can result in changes in DNA sequence which will in turn affect gene expression. This also influences the cell’s proliferation, dysfunction, and death over its lifetime.
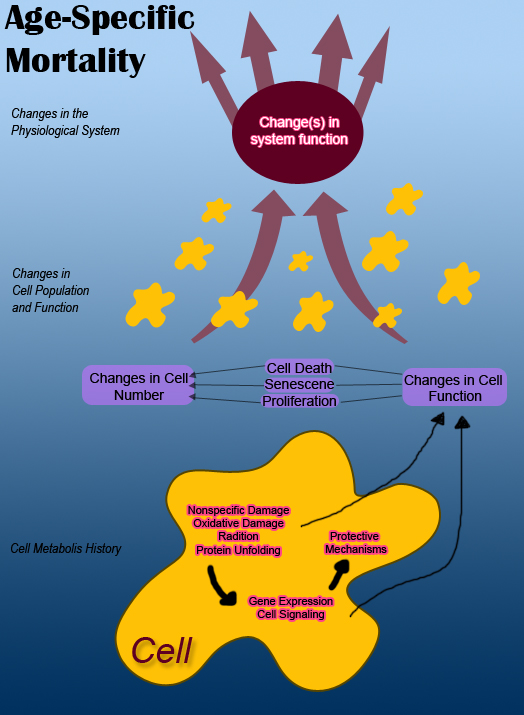
All these topics are related to each other and to the process of aging. Dysfunction of a system occurs due to the changes in cell number and their function. What causes the changes in cell number and function is caused by the cell’s metabolic history that involves mainly nonspecific damage and changes in gene expression. The result from these changes in the metabolic history results in changes in cell number and function. The important and contributing factors of a cell’s metabolic history which influences aging are nonspecific damage and changes in gene expression and signaling pathways.
Nonspecific Damage
[edit | edit source]Different types of nonspecific damage done to the organism contribute to the loss of cell function and dysfunction. Some damages include oxidative damage, radiation, or chemical reactions. The nonspecific damage end up damaging and hindering molecules as time passes on. The cell tries its best to survive and to protect itself against the damage. The cell initiates damage repair in cases where it can fix a misfolded protein but damages such as a fixation of a DNA mutation cannot be resolved. Such nonspecific damages that are also irreversible will greatly impair the cell and its function. The question arises whether the damage done to the cell affects outputs of the system enough for aging.
Gene Expression and Cell Signaling
[edit | edit source]In regards to nonspecific damage, it can affect gene expression and cell signaling of the cell. What the gene expresses that was affected by nonspecific damage will also affect its outputs of the system that affects mortality during normal aging. There is evidence of there being a great amount of changes in gene expression that may occur during normal aging but their contribution in the aging process will vary across systems and overall the organism.
Metabolic Control Analysis and Aging
[edit | edit source]Metabolic Control Analysis (MCA) – an experimental approach developed to understand how control is distributed within metabolic pathways and networks
In order to determine what is involved with the process of aging, there must be a set limit on systems being analyzed and the measurable variables being observed. Once these factors are defined and limited, the physiological system in question can be tested and manipulated so a relationship between said variable and the step it is controlling can be seen. The extent of the variable’s control will also be determined during this experiment of MCA. With the use of MCA, there has been powerful mathematical formalism developed in order to see the entire system and the following: extent of control to be determined quantitatively, uncovering of all controlling sits, and influence of controlling steps. Though it has been shown that extreme and large changes in biochemical pathways and mechanisms can increase or decrease life span, there is not enough evidence that shows they influence normal aging. Comparing alongside metabolic pathways and aging, MCA can tell one the important information of steps in controlling of a particular process in a system.
Application of MCA to Aging
[edit | edit source]The process of MCA and it being applied on a system needs to be conducted a certain way in order to obtain results. Since the topic is the aging of organisms, this requires mortality readings that relate to organismal aging. As mentioned in the previous section, small changes to factors of a system can be made and by utilizing mathematics, quantifiable results can be obtained. The read out of the mortality needs to be measured in response to the small changes made in the system. Recall that those changes in a factor are due to the belief that it may control aging. In order for a factor to affect aging, it must alter the corresponding morality rate and contribution to aging. The MCA experiments are conducted with populations of a diverse group of animals ranging from flies to mice. The mortality readout can then be measured and compared to see if there is a similarity across the species.
In the graph shown above, the morality readout is shown by plotting it against the variable in question for aging that has been both increased and decreased. The shaded dark blue area indicates how the variable alters in normal aging. The graph also displays different situations for normal aging. The effect of the variable can be decreased or increased which affects mortality as well. This is an example of a graph that can be plotted after an MCA experiment to showcase one’s findings and results. The main idea is that if the right variable is chosen that does affect normal gaining, the curve of mortality will have a slope that is measurable.
Role of Chromatin in Aging
[edit | edit source]Chromatin is first formed in an organism during embryogenesis. This genetic material is susceptible to modification via DNA methylation and different types of histone modification; these changes do not affect the nucleotide sequence of DNA. The accumulation of changes over an organism's lifetime results in stochastic, random, structure. In an experiment with Danish twins, it was observed that variations in relatives is a result of genetic shift from chromatin changes. This supports the idea that One theory cites an experiment with Danish twins and proposes that aging occurs as a result of the accumulation of changes in the chromatin structure(2). There are several proteins associated with chromatin modification. These proteins directly affect transcription regulation, but also affect the structure and the expression of the genes in chromatin.
CDKN2a (p16INK4a)
[edit | edit source]CDKN2a regulates the retinoblastoma protein, which suppresses tumors, by preventing CDK4/CDK6 from phosphorylating retinoblastoma protein. Increased expression of CDKN2a is associated with cellular senescence and aging, which is linked to increased levels of stress on cells. Because of these associations, it has been suggested to use this protein as a biomarker for aging. In an experiment with murine BubR1 haploinsufficiency, it was found that subjects that eliminated CDKN2a-positive cells had an extended lifespan and less age related degeneration. The experiments also indicate that by removing CDKN2a-positive cells in old mice improved deterioration due to aging and prolonged these effects. CDKN2a expression is controlled by trithorax (TrxG) and polycomb (PcG), which turn the gene on and off respectively.
SIRT1 and SIRT6
[edit | edit source]SIRT1 and SIRT6 , both NAD+-dependent lysine deactylases, play roles in DNA repair and aging. A homologous protein to SIRT1 and SIRT6, Sir2 has been found and experimented on in yeast. This protein silences rRNA and telomeres. Without the expression of this gene, then rDNA arrays are removed from the genome to form circular DNA and the lifespan of the yeast declines. Similarly, SIRT1 and SIRT6 affect the cell's response to DNA repair and stress levels. These two proteins influence the aging process through DNA repair processes and transcription regulation.
When the DNA undergoes oxidative damage, SIRT1 is dispersed to all repair sites where H1K26 is deacetylated to increase DNA repair. However, when SIRT1 is recruited to damaged areas of the genome, ataxia telangiectasia-mutated pathway activation is required, which deacetylates Nijmegan breakage syndrome protein (NBS1), which controls the pathway to DNA repair, but also damage-dependent transcriptional deregulation. Therefore, while SIRT1 maintains DNA, it also plays a critical role aging. Increased SIRT1 expression does not increase an organism's lifespan, but it does increase its tumor resistance, improve its metabolism and prevents diabetes. SIRT1 also plays a role in stimulating non-histone proteins such as p53 (tumor suppressor gene), FOXO (transcription box), and Ku70 (DNA repair factor).
An overexpression of SIRT6, however, does increase the lifespan of the mouse (especially in males). SIRT6 is a regulatory protein that affects the nuclear factor kappa-light-chain-enhancer of activated B-cells-dependent inflammatory response (NF-kB) by attaching to its active site and inhibiting its transcription. Therefore, decreased SIRT6 expression results in increased NF-kB response. Humans lacking SIRT6 are more susceptible to attack by DNA-attacking agents. SIRT6 is also involved in homologous recombination, regulating response to DNA damage, and promote aging by increasing telomere malfunction.
Conclusion
[edit | edit source]Overall, the development of MCA methods has helped immensely the degree of understanding what influences and affects the normal aging process. MCA methods should be applied alongside with experimental models testing for aging because it will allow us to address the possible factors that affect aging.
Notes
[edit | edit source]References
[edit | edit source]- Murphy, Michael P. and Partridge, Linda. “Toward a Control Theory of Aging.” ["http://www.ncbi.nlm.nih.gov/pubmed/18318658"], 'Annual Review of Biochemistry', 2008.
- http://www.annualreviews.org/doi/pdf/10.1146/annurev.biochem.77.061206.171059
- O'Sullivan, Roderick and Jan Karlseder. "The Great Unravelling: Chromatin as a Modulator of the Aging Process". ("http://www.sciencedirect.com/science/article/pii/S0968000412001132"), 'Trends in Biochemical Sciences', 1 November 2012 (Vol. 37, Issue 11, pp. 466-476)

