Structural Biochemistry/Protein function/Heme group
Red blood cells, or erythrocytes are by far the most numerous blood cells. Each red blood cell contains hemoglobin which is the iron-containing protein that transports oxygen from the lungs to other parts of the body. In hemoglobin, each subunit contains a heme group; each heme group contains an iron atom that is able to bind to one oxygen molecules. Since hemoglobin consists of four polypeptide subunits, two alpha chains and two beta chains, and each subunit contains a heme group; each hemoglobin protein can bind up to four oxygen molecules.
General information
[edit | edit source]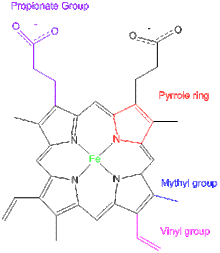
The prosthetic group consists of an iron atom in the center of a protoporphyrin which is composed of four pyrrole rings that are linked together by a methene bridge, four methylene groups, two vinyl groups and two propinoic acid side chains. Each pyrrole ring consists of one methyl group. Two of the pyrrole rings have a vinyl group side chain, while the other two rings have a propionate group independently. Heme proteins have some iron-porphyrins such as heme a, heme b, heme c, heme d, heme d1, heme o, etc. They are constituted by tetrapyrrole rings but differ in substituents. For example, heme o contain four methylene groups while heme a contain three methylene groups, the rest structure are similar between two groups. The difference between hemes assigned each of them different functions.
Heme of hemoglobin protein is a prosthetic group of heterocyclic ring of porphyrin of an iron atom; the biological function of the group is for delivering oxygen to body tissues, such that bonding of ligand of gas molecules to the iron atom of the protein group changes the structure of the protein by amino acid group of histidine residue around the heme molecule. A holoenzyme is defined to be an enzyme with its prosthetic group, coenzyme, its cofactor, etc. Therefore an example of a holoenzyme is hemoglobin with its iron-containing heme group.

Heme A
[edit | edit source]Heme A is a bimolecular heme that is made up of of macrocyclic ligand called a porphyrin, chelating an iron atom. Heme A differs from Heme B in that it contains a methyl side chain at a ring position that is oxidized to a formyl group and hydroxyethyfarnesyl group. Moreover, the iron tetrapyrrole heme will be attached to a vinyl side and an isoprenoid chain. Heme A is known to be relatively comparable to Heme O since both include farnesyl.
Heme B
[edit | edit source]Heme B is present in hemogoblin and myogoblin. Typically, heme B is binded to apoprotein, a protein matrix executed with a single coordination bond between the heme iron and amino-acid side-chain.
The iron contained in heme B is bounded to four nitrogens of the porphyrin and one electron donating atom of the protein, which puts it in a pentacoordinate state. The iron turns into a hexacoordinate when carbon monoxide is bounded.
Heme C
[edit | edit source]Heme C differs from heme B in that the two vinyl side from the heme B are substituted with a covalently thioether linkage with the apoprotein. Because of this connection, heme C has difficulty dissociating from holoprotein and cytochrome c.
Heme C functions a crucial role in apoptosis because some molecules of cytoplasmic cytochrome c must contain heme C. As a consequence, this will lead to cell destruction.
Heme D
[edit | edit source]Heme D is another form of heme B. Instead, the hydroxylated propionic acid side chain forms a gamma-spirolactone. Heme D reduces oxygen in water of bacteria with a low oxygen tension.
Coordination
[edit | edit source]Heme is a porphyrin that is coordinated with Fe(II). One of the most important classes of chelating agents in nature are the porphyrins [1]. A porphyrin molecule can coordinate to a metal using the four nitrogen atoms as electron-pair donors. In the body, the iron in the heme is coordinated to the four nitrogen atoms of the porphyrin and also to a nitrogen atom from a histidine residue, one of the amino-acid residues in hemoglobin) of the hemoglobin proteins. The sixth protein coordination site, around the iron of the heme, is occupied by O2 when the hemoglobin is oxygenated. The heme group is nonplanar when it is not bound to oxygen [2]. The iron is pulled out of the plane of the porphyrin, towards the histidine residue to which it is attached. This nonplanar configuration is characteristic of the deoxygenated heme group, and is often referred to as being "domed shape" [2]. When the Fe heme group binds to an oxygen molecule, the porphyrin ring adopts a planar configuration and hence the Fe lies in the plane of the porphyrin ring [2]. Oxygenated and deoxygenated conformation of Hemoglobin
As may be seen in the figure, the left shows representations of electron-density clouds of the de-oxygenated heme group, depicted in pink, and the attached histidine residue which may be seen in light blue [2]. These regions of electron density repel each other, and the iron atom in the center is drawn out of the plane. The non planar shape of the heme group is represented by the bent line. The right image depicts the electron-density clouds of the oxygenated heme group, shown in pink, the attached histidine residue in light blue, and the attached oxygen molecule which is shown in gray [2]. The oxygenated heme assumes a planar configuration, and the central iron atom occupies a space in the plane of the heme group which is depicted by a straight red line [2].
Heme Group Shape
[edit | edit source]The shape change in the heme group also has important implications for the rest of the hemoglobin protein. When the iron atom moves into the porphyrin plane upon oxygenation, the histidine residue to which the iron atoms is attached to is drawn closer to the heme group. This movement of the histidine residue then shifts the position of other amino acids that are near the histidine [2]. When the amino acids in a protein are shifted in this manner by the oxygenation of one of the heme groups in the protein, the structure of the interfaces between the four subunits is altered [2]. So when a single heme group in the hemoglobin protein becomes oxygenated, the whole protein changes its shape [2]. In the new shape, it is easier for the other three heme groups to become oxygenated. The binding of one molecule of oxygen to hemoglobin enhances the ability of hemoglobin to bind more oxygen molecules. This property of hemoglobin is known as "cooperative binding" [2].
Association Constant
[edit | edit source]Association constant is the constant at which the bonding affinity between two different molecules, the substrate and the product, is at stable equilibrium. An example of such a bonding constant occurs in the hapten-antibody interaction.
Dissociation Constant
[edit | edit source]Dissociation constant is the quantifiable constant in which a compound, molecule, or ion dissociates. A type of dissociation constant is acid dissociation constant. This constant is used to calculate the occurrence of a weak and strong acid dissociation.
Function
[edit | edit source]The Heme group gives myoglobin and hemoglobin the ability to bind oxygen because of the presence of iron atom. It also contributes to the red color found in muscles and blood. Each heme group contains an iron atom that is able to bind to one oxygen (O2) molecule. Each hemoglobin protein can bind four oxygen molecules. The iron atom, usually in the ferrous oxidation state (Fe2+), lies between four pyrrole rings but slightly bends away from the plane (0.4 Angstrom from the plane). The iron ion has two extra binding sites called the fifth and sixth coordination sites on each side of the protoporphyrin plane. Usually, the fifth coordination binds with proximal histidine where the sixth coordination binds to an oxygen. When oxygen binds to iron, the iron becomes slightly smaller allowing it to move into the plane of the porphyrin ring. A distal histidine binds to oxygen to make sure reactive oxygen is not released. The distal histidine will not allow the release of oxygen when the Iron is in the 3+ state.
Crystal structure of apo heme oxygenase-1
Mechanistic Functionality
[edit | edit source]The Iron atom is too large in size to fit perfectly inside the porphyrin ring, and sits outside the ring by 0.4 Angstroms. However, upon binding oxygen, the Iron radius shrinks, facilitating a planar alignment with the porphyrin ring. This change causes the proximal histidine bound to the Fe atom to be pulled up and cause a structural change to the alpha helix attached to the histidine residue. This alpha helix's carboxyl terminus interacts with the other alpha-beta dimer, creating a total conformational change in the overall protein. The conformational change facilitates an increased affinity for oxygen, which is shown by a transformation from the T to R state in hemoglobin. The changes that occur in blood upon oxygenation and deoxygenation are visible not only at the microscopic level but also at the macroscopic level. It has been known that blood in the systemic arteries is red-colored while blood in the systemic veins is blue. The blood in the systemic arteries is oxygen-rich, having just traveled from the lungs to the heart and then being pumped throughout the body to deliver its oxygen to the body's cells [2]. The blood in the systemic veins, on the other hand, is oxygen-poor. It has unloaded its oxygen to the body’s cells and must now return to the lungs to replenish the supply of oxygen [2]. Hence, a simple macroscopic observation such as noting the color of the blood, can tell us whether the blood is oxygenated or deoxygenated.
